The Management of Hyperosmolar Hyperglycaemic State in Adults
One of our expert writers has created this bespoke sample nursing dissertation that shows the quality that is guaranteed with every nursing paper ordered. Secure your academic success and place an order today or view our services.
This sample is part of a set:
- Nursing Dissertation Topic with With Titles (Undergraduate 2:1)
- Nursing Dissertation Proposal (Undergraduate 2:1)
- Full Nursing Dissertation (Undergraduate 2:1)
Table of Contents
- Abstract
- Chapter 1: Introduction
- Chapter 2: Normal glucose metabolism and the pathophysiology of diabetes mellitus.
- Chapter 3: Hyperglycaemic diabetic emergencies (HHS and DKA).
- Chapter 4: Literature review methodology
- Chapter 5: Results of the literature search and critique of selected articles.
- Chapter 6: Discussion- implications for practice.
- Chapter 7: Summary and conclusions
- References
- Appendices
- Appendix 1: Preferred Reporting Instrument for Systematic reviews and Meta Analyses (PRISMA) diagram illustrating the article selection procedure.
- Appendix 2: Table 2A, Non-selected studies
- Appendix 3: Table 3A Data extraction (selected studies)
- Appendix 4: The JBDS HHS care pathway
Abstract
HHS is a rare complication of DM and differs significantly from DKA, which is a second type of diabetic emergency (Scott, 2015). Although relatively rare, the mortality associated with HHS is significantly higher than that associated with DKA, which is estimated at between 3-5% (Scott, 2015; Misra & Oliver, 2015). Precise mortality figures for HHS vary because the condition arises mainly in elderly patients, often affected by several other comorbidities and, in addition, no population based studies have been done to obtain an accurate, specific HHS mortality rate (Blouin, 2012). The pathological changes that are responsible for the development of HHS, as opposed to DKA, are not understood, although certain risk factors appear to act as triggers. These include age, ethnicity, trauma, infection and surgery (reviewed by Kitabchi et al., 2009). DKA is a hyperglycaemic crisis that arises when there is a complete absence of insulin, in type 1 DM (described below). It is characterised by the presence of ketones in blood and urine, which give rise to ketoacidosis. HHS, on in contrast, is generally associated with type 2 DM, and, because there is typically some insulin present, the process that generates ketones is inhibited, thus ketoacidosis is evident unless there is some DKA present (Chiasson et al., 2003).
The cost of treating diabetic emergencies such as DKA and HHS is significant because they inevitably require hospital admission and discharge home from emergency departments is almost always impossible (Centres for Disease Control and Prevention, 2007; Elliott, at al., 2014). Elliott et al., (2014) report that, in a National diabetes audit (Health and Social Care Information Centre (HSIC), 2013), 6141 people with type 1 DM were admitted to hospital in the UK in 2010-2011, each requiring treatment estimated by the NHS information service (2011) to cost approximately £900 each, equating to approximately £5.5 million in total. It is worth acknowledging that these estimates are now somewhat out of date, and more recent estimates for DKA treatment are approximately £980 per admission (NICE 2016a).
Given the importance of achieving an accurate diagnosis, that discriminates between DKA and HHS, there is significant need for nurses to possess evidence based knowledge to inform their care of HHS patients, and ensure appropriate treatment is delivered.
The NMC (2015) demands the nurses have knowledge of, and understand, the evidence based treatment options available to HHS patients. These patients, when admitted to emergency departments, often have impaired mental function because of the pathophysiology of the condition (discussed below) or are comatose, as a result of the pathophysiological effects of hyperosmolarity on the brain (Guisado and Arieff, 1975). In such situations, nurses are required to assume the role of patient advocate. This can only be achieved safely, and within the competence limits demanded of individual practitioners by the NMC (2015), with sufficient knowledge of the evidence base supporting clinical decisions. Therefore, it is incumbent upon nurses treating HHS patients to be aware of the best evidence base supporting specific interventions and challenge practice that is not supported by the most recent, valid, research evidence. This dissertation reviews the current evidence base for the treatment of HHS in adults, in the UK.
Chapter 1: Introduction
The dissertation is a systematic review, defined by Bettany-Saltikov (2012:5) as “A summary of the research literature that is focused on a single question”. It will comprise a background and introduction’, ‘methods’, ‘results’, ‘discussion’ and ‘conclusions’, as recommended by Walliman, (2004) and Greetham (2014). This chapter provides an introduction to the dissertation, states its aims and objectives, and outlines the content of the following chapters.
The abstract summarises the subject of the literature review, and a rationale for this study into the treatment of hyperosmolar hyperglycaemic syndrome (HHS). HHS is a complication of diabetes mellitus (DM) that, in common with diabetic keto acidosis (DKA) and hypoglycaemia, is a major cause of admission by diabetic patients to emergency departments (Elliott et al., 2014). Unlike DKA, which is treated according to National Institute for Health and Care Excellence (NICE) guidelines (NICE, 2015a), there are no NICE guidelines available in the UK for the treatment of HHS. Instead, the HHS guidelines produced by the Joint British Diabetes Societies (JBDS) Inpatient Care Group (Scott, & Claydon, 2012), are recognised by the National Health Service (NHS) as the ‘gold standard’ for the treatment of HHS in the UK.
If you need assistance with writing your dissertation, our professional Dissertation Writing Service is here to help!
Find out moreAlthough DKA and HHS share similar pathophysiology, there are crucial differences that impact upon treatment. Failure to treat HHS appropriately (for example, by treating it as for DKA), can be fatal, and cases in which patients have either died or been permanently have been reported (Blouin, 2012; Gouveia & Chowdhury; 2013).
In view of the pathophysiological basis for HHS and DKA, it is necessary, before evaluating treatment regimens for HHS, to provide an overview of their primary cause, which is DM, and discuss in more detail the clinical features of DKA and HHS, highlighting common features, but crucially, identifying differences. This discussion is provided in chapters 2 and 3 that outline the pathophysiology of DM, and hyperglycaemic crises, respectively.
The research question was formulated, and relevant literature searched, as described in chapter 4 ‘methods’. The research question was then applied to guide the search strategy of three major health databases. Chapter 4 summarises the search strategy applied in sufficient detail for it to be reproduced. The process of article selection is summarised in Appendix 1, and, to further support transparency and the prevention of reviewer bias, non-selected studies are listed in Table 2A, Appendix 2. The selected articles are included in the reference list, indicated by an asterisk.
Chapter 5 presents key points arising from a critical appraisal of the literature retrieved. A synthesis of the articles’ findings, and comparison of the recommendations made to those of the JBDS (Scott and Claydon, (2012), see Appendix 4) was derived through the application of data extraction table, summarised in Table 3A in Appendix 3. Chapter 6 revisits the research question and considers the implications of the information gained for nursing practice, emphasising the role of nurses in ensuring patients suffering from HHS receive appropriate, evidence based treatment as required by the Nursing and Midwifery Council (NMC, 2015). The potential psychosocial effects of HHS, as a serious complication of diabetes, are reflected upon in this chapter and the role of nurses in preventing patients from experiencing similar crises in future is considered. Chapter 7 summarises key points and revisits the dissertations aims and objectives, while considering the strengths and weakness of the study in meeting them.
1.0 Rationale, Aims and Objectives
The current knowledge base available to inform nurses and support the delivery of the highest quality evidence based care possible to HHS patients appears to lack consensus. As noted previously, unlike DKA, NICE has yet to produce agreed evidence based guidelines for the treatment of HHS. Furthermore, a search of the Cochrane Database of Systematic Reviews (Cochrane Library, 2017) by the author revealed a lack of any systematic reviews or meta-analyses that could provide those treating HHS patients with a consensually accepted evidence base to support clinical decision making.
Arguably, nurses could simply assume that, by following the JBDS HHS care pathway (Scott and Claydon, 2012), or a Trust protocol based upon it (if available), their professional obligations to the NMC (2015) will be met. This dissertation questions that assumption, on the basis that the JBDS HHS protocol is 5 years old, and there may be more recent alternatives available based on additional research evidence. The literature search aims to retrieve any HHS protocols published after 2012, and compare their recommendations to those of the JBDS highlighting any variations in HHS management and identifying interventions based upon recommendations that are controversial or lack consensus. In addition, the search aims to locate any new primary research that could potentially inform the treatment of patients with HHS.
The role of nurses in striving to improve the quality of patient care is not only embodied by the NMC (2015), but is a key component of the nursing contribution to government and healthcare policies such as The Five Year Forward View (NHS England, 2014), that aim to eradicate poor practice following recommendations made by public inquiries such as The Francis Report (Francis, 2013) and the Bristol Royal Infirmary Inquiry (Department of Health (DH) 2002). These inquiries have compelled contemporary healthcare policymakers to focus upon achieving quality and excellence, expounded by the current NHS constitution (DH, 2012). It is anticipated that this dissertation will communicate the most current information regarding HHS treatment regimens, and their rationales to nurses in an accessible form, and, together with a critique of the literature retrieved, support nurses’ capacity to deliver high quality, evidence based care for HHS patients.
In summary, the aims of this dissertation are to enable nurses caring for HHS patients-
1) To gain awareness of the most recent, evidence based treatment protocols and recommendations made for the treatment of HHS.
2) To compare the above information with the recommendations made by the JBDS (Scott and Claydon, 2012), which apply in the UK in the absence of NICE guidelines relating to this condition, and be suitably informed to challenge inappropriate treatment.
CHAPTER 2: Normal glucose metabolism and the pathophysiology of diabetes mellitus.
2.0. Background
According to recent statistics, there were over 3.5 million diagnoses of DM in 2015 (Diabetes UK, 2016). There are two types of DM; type 1 and type 2. Type 1 DM accounts for approximately 5-10% of DM cases (World Health Organization (WHO) 2013) and arises from the production of insufficient insulin, a hormone which plays a central role in controlling blood glucose concentrations within physiologically desirable limits, as described below. Type 1 DM typically develops over a short period of time, and is more common in children (Atkinson et al., 2014). The pathology of type 1 diabetes is discussed in more detail in part 2.1 (ii), but to summarise, consensus opinion is that Type 1 DM is caused by environmental factors (Maclaren and Atkinson, 1992; The Environmental Determinants of Diabetes in the Young (TEDDY) Study Group, 2008), combined by genetic predisposition (Bluestone et al., 2010; Howson et al., 2011; Atkinson et al., 2014)
Type 2 DM is a chronic metabolic disorder, that, in contrast to type 1 DM, is largely caused by relative insulin deficiency, which predominantly arises from insulin resistance but, if not managed, can lead absolute insulin deficiency (Muoio & Newgard, 2008). 90% of all DM is type 2 (World Health Organization (WHO, 2013) and, although there appears to be some genetic and/ or epigenetic predisposition, (Anderson et al., 2016; Sommese et al., 2017 respectively), the condition is regarded by the WHO (2013) as a lifestyle related disease. This classification by the WHO reflects substantial research that has identified lifestyle as a source of modifiable risk factors (such as diet and exercise) that can significantly reduce the risk of developing type 2 DM (Godino et al., 2916; Johansen, et al., 2017; Rapp, et al., 2017; Gilis-Januszewska et al., (2017) and several others). Therefore, unlike type 1 DM, type 2 disease can be prevented and managed by regular exercise and the adoption of diets that are high in fibre, low in sugar and low in high glycaemic index foods, as well as low in saturated and trans polyunsaturated fat (as advocated by NHS UK, 2017; Diabetes UK, 2017, NICE, 2011 and NICE 2015b). Should lifestyle interventions fail to control hyperglycaemia, treatment with glucose lowering drugs such as metformin is recommended, with supplementation by insulin, should this medication (combined with other glucose lowering drugs) prove ineffective (NICE, 2015b)
The rates of type 2 DM have been rising steadily since the 1960’s in line with the rise in obesity (Olokoba et al., 2012). Significantly, Rosenbloom, (2010), Atkinson (2014) and Canarie (2007) report that rates of type 2 DM appear to be increasing in young people, and Muoio and Newgard (2008) comment that type 2 DM is projected to affect 300, 000 million people globally, by 2020. The downward demographic shift in the population afflicted by type 2 DM and its increased incidence are causes for concern, given the complications associated with the disease, which include neuropathies, nephropathies, cardiovascular disease lower limb amputations and depression (Hill, 2008, Dolan et al., 2017; Alva et al., 2015; Almdal et al., 2004 and many others).
Epidemiological evidence suggests that type 2 DM and associated comorbidities shorten life expectancy, although this trend appears to be reducing with better treatment, education and the adoption of healthier lifestyles to manage the condition and its complications (Mulnier et al., 2006; Astrup, 2001; Nwaneri et al., 2013). However, Kitabchi et al.’s (2001) review asserts that mortality due to type 2 DM cannot be assessed accurately due to the significant influence of comorbidities and lack of information presented on death certificates.
Relative insulin deficiency (in type 2 DM) and complete insulin deficiency (type 1 DM) cause the failure of homeostatic mechanisms that maintain normal fasting venous plasma glucose within the physiological limits of 3.9-6.1 mmol/L, (Barrett, 2010). Glucose, a product of enzymic digestion of sugars and carbohydrates in the intestine, is an essential physiological energy source, and crucial for the optimal functioning of the nervous system and brain (Kisiel and Marson, 2009). Two hormones, insulin and glucagon, regulate circulating glucose levels.
Normally, glucose released by digestion enters the blood, reaching a peak of 7-9 mmol/L, 30-60 minutes after a meal (Higgins, 2001) and stimulating insulin production. Insulin is a polypeptide hormone produced by the β cells located within the islets of Langerhans in the pancreas. In addition to promoting cellular glucose uptake and the storage of excess glucose as glycogen, insulin promotes protein synthesis and inhibits the synthesis of glucose from other substrates, such as fatty acids (Barrett 2010; Williams & Pickup, 2004).
Once released into the plasma, activated insulin has a half-life of 5 minutes (Pørksen et al., 1997) to 15 minutes (Hardern and Quinn, 2003), depending upon the method used for measurement. During circulation, insulin binds to its specific receptors on the surface of target cells (such as those in muscle, brain and liver, shown in Figure 1) and exerts its effects. Intracellular glucose, increased following insulin binding to its cell surface receptor, is phosphorylated by the enzymes hexokinase and glucokinase. Glucose 6-phosphate is then polymerised into glycogen by glycogenesis (Barrett 2010; Williams & Pickup, 2004).
Glycogen is large polysaccharide that acts as a storage compound to release energy in the form of adenosine tri-phosphate (ATP) from the oxidation of glucose, supplied by glycogenolysis, when physiological energy demand exceeds supply (such as in periods of exercise or starvation). In these situations, when plasma glucose is reduced, the α cells in the islets of Langerhans secrete glucagon which stimulates glycogenolysis, and gluconeogenesis from amino acids (Kisiel and Marsons, 2009; Barrett, 2010; Williams & Pickup, 2004).
Figure 1 provides a highly simplified summary of glucose metabolism and the key processes that are affected by insulin deficiency (highlighted by red text, crosses and arrows), and which are discussed below.
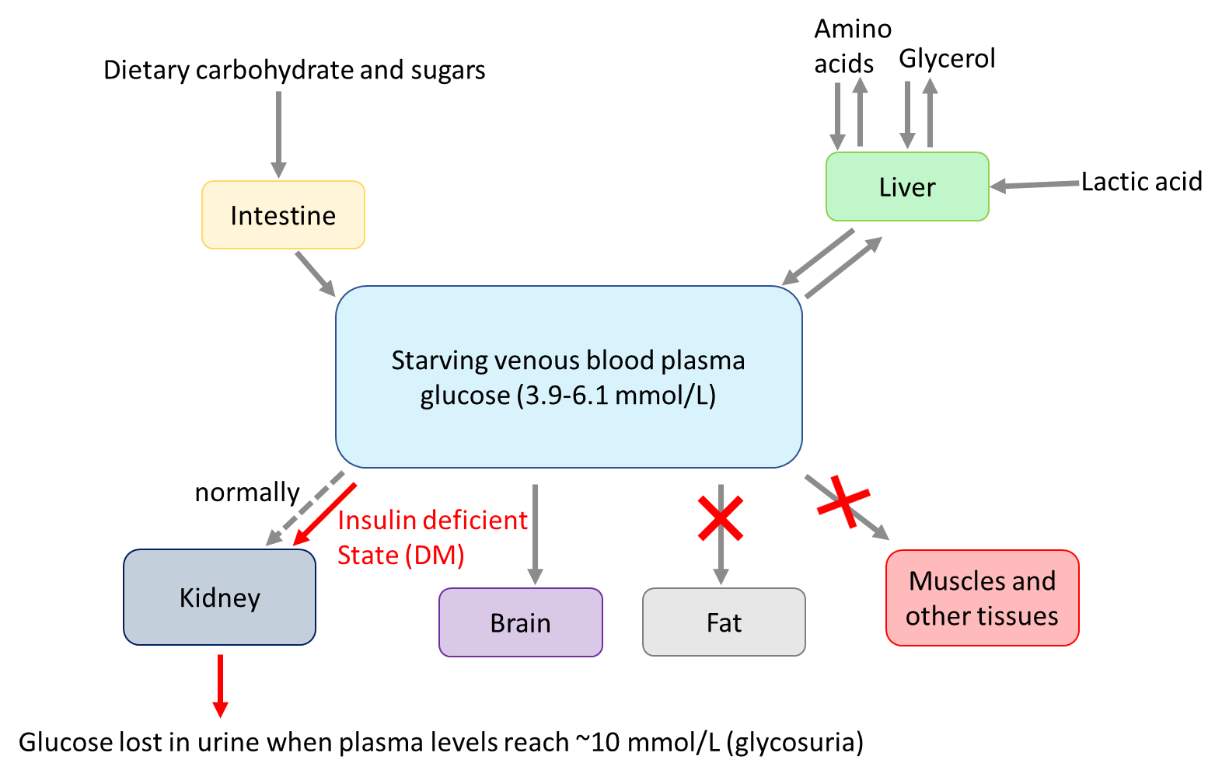
Figure. 1: Simplified schematic diagram providing an overview of glucose metabolism. Processes that are affected by insulin deficiency are highlighted by red text, crosses and arrows and are discussed below. (Source: The author, adapted from Barrett, 2010)
2.1 the pathogenesis, of DM
(i) Type 2 DM,
This condition, in common with type 1 DM, arises from failure of glucose homeostasis, but unlike type 1 DM, appears to be caused by a complex interaction between chronic over nutrition (often associated with obesity), and genetic susceptibility, which can give rise to insulin resistance (IR) (Williams and Pickup, 2004). These authors summarise the effects of IR, in causing cellular failure to respond to insulin by taking up glucose from blood plasma. Inhibition of the normal process of glucose absorption allows glucose to accumulate in plasma, leading to hyperglycaemia. As the condition progresses, the normal negative feedback loop that maintains blood glucose homeostasis is replaced by a positive feedback mechanism that causes the β cells to secrete even more insulin in an attempt to reduce high circulating glucose levels. This leads to high concentrations of circulating insulin that fails to exert any physiological effect (IR) and which appears to eventually lead to β cell failure (Reaven et al., 1976). At this point, patients may develop type 2 DM, and may require drug treatment to stimulate residual β cell activity (typically, these include progressive treatment regimens of metformin and gliazones, supplemented with sulphonylureas and finally insulin when β cell function has ceased (NICE, 2015b).
Historically, IR has been associated with obesity, but until recently research has been unable to identify the physiological basis for the association made between type 2 DM and obesity identified by numerous research studies (see Guh et al., 2009 for a review). Although beyond the scope of this dissertation, contemporary research has identified several potential contributory factors to IR development, many of which are attenuated by lack of exercise and excess weight. These include inflammatory mediators, adipokines, ceramides, metabolic liver and skeletal muscle overload (reviewed by Muoio, & Newgard, 2008).
Hill’s (2009) review reports that IR does not necessarily lead to type 2 DM, and may go undetected. The distinguishing point at which type 2 DM develops, however, appears to be β cells failure, combined with predisposing genetic factors (Reaven et al., 1976; Muoio & Newgard, 2008; Williams and Pickup, 2004).
Type 2 DM typically develops slowly, over a period of years, with the gradual appearance of symptoms. A proportion of the population may be classed as ‘prediabetic’, and in the process of developing IR, and in addition it is estimated that many people have the condition, remain asymptomatic and undiagnosed (Simmons et al., 2012). The main symptoms of DM include hyperglycaemia, glycosuria (in well-developed type 2 DM, as the blood plasma glucose concentration exceeds to renal threshold for reabsorption, leading to the loss of glucose in urine), polyuria due to the contribution if the glucose to plasma osmolality, abnormal thirst due to dehydration caused by high urine output, weight loss due to fat and protein metabolism, slow healing of wounds, skin itching, frequent yeast infections, fatigue and feeling hungry (Clancy & McVicar, 2009; Diabetes UK, (2017).
(ii) Type 1 DM
Current research evidence, reviewed by Atkinson et al., (2014) suggests that insulin deficiency, evident in type 1 DM, is caused by autoimmune destruction of insulin secreting cells in the pancreas). The main risk factor is family history of type 1 DM. Although the condition cannot be prevented or cured, it can be managed by insulin replacement therapy, exercise and by adopting a ‘diabetic’ diet in which the intake of glucose is controlled (NICE, 2015). Type 1 DM can be distinguished from type 2 by the presence of autoantibody screening which identifies anti-islet autoantibodies in the blood (Winter & Pitman, 2013).
This type of DM has been subject of much investigation over the past decade, improving knowledge of the condition’s pathogenesis, predictive risks and epidemiology (Atkinson et al, 2014). Despite this concerted research effort, type 1 DM (unlike type 2) appears resistant to prevention or cure. An autoimmune mediated destruction of pancreatic β cells is commonly regarded as the primary cause, but this is contentious; approximately 10% of type 1 DM patients do not possess self-reactive antibodies to pancreatic beta cells, and this subset of individuals are classified as having idiopathic type 1B DM, without any clear pathogenesis (Eisenbarth, 2007; Gianani et al., 2010). Atkinson et al., (2014) assert that the aetiology of type 1 DM has a strong genetic influence, with over 40 genetic loci identified to date (reviewed by Concannon et al., 2009).
Type 1 DM has many symptoms in common with type 2 DM, but crucially their appearance is more rapid and acute. For example, the slow healing of cuts is unlikely to provide a symptomatic indicator of type 1 DM because the effects of total insulin deficiency precipitates severe pathophysiological changes such as excessive thirst and glycosuria very rapidly (Clancy & McVicar, 2009).
Chapter 3: Hyperglycaemic diabetic emergencies (HHS and DKA).
3.0 Overview
Dhatariya (2014) states that DKA and HHS are two of the most common metabolic emergencies, both of which can be fatal if not treated appropriately. Gouveia and Chowdhury (2013), for instance, report a case in which clinical differentiation between DKA and HHS was not made accurately, leading to the patient with HHS receiving a treatment regime suitable for DKA, with catastrophic consequences. These authors point out that differentiating between the two conditions can be difficult, but the importance of accurate diagnosis before commencing treatment cannot be over emphasised. As the HHS patient’s advocate (as required by the NMC (2015) if the patient cannot communicate), it is important that their emergency nurse has sufficient knowledge to recognise the diagnostic differences between the two conditions, arising from their differing pathophysiology, and understands the importance of providing appropriate treatment. This chapter outlines the main diagnostic indicators of DKA and HHS, compares the typical biochemical profiles of each condition, and highlights recommendations that appear to lack consensus amongst specialist clinicians, based upon the authors review of the literature.
3.1 HHS
Much of the older literature reflects the general consensus that HHS is largely associated with elderly patients and/ or those suffering from type 2 DM (for example, Gill and Alberti, 1985; Paterson, 1990; Wachtel, et al., 1987; Mazze 1985, and many others). More recently, however, it has been recognised that the incidence HHS is not necessarily restricted to the elderly, and can arise as a complication of type 2 diabetes in children and adolescents (Fourtner et al., 2005 and Rosenbloom, 2010).
Furthermore, it is now acknowledged that HHS may represent the first signs of previously undiagnosed DM (Scott, 2015; Kitabchi, et al, 2009). HHS has a slower onset than DKA; typically, HHS develops over a period of days, whereas the onset of DKA is within hours (Chiasson et al., 2003). Kitabchi et al.’s (2009) review suggests that the prolonged accumulation of the metabolic anomalies and dehydration that arise in HHS are a key feature of the condition, but HHS has yet to receive a consensual definition (Scott, 2015). These issues pose difficulties when distinguishing between HHS and to DKA, and thus influence important clinical decisions regarding the most effective, safe treatment. HHS is associated with very high levels of blood glucose (with a mean value 55 mmol/L (Gill and Alberti, 1985), well in excess of those typically observed in DKA. The presence of plasma ketones has been used as a key diagnostic criterion, because residual pancreatic β cell function in type 2 DM produces small quantities of insulin that inhibits the metabolic processes that generate ketones from fatty acid metabolism (Scott, 2015; Kitabchi et al., 2009; Williams & Pickup 2004). Therefore, in HHS ketones such as beta-3-hydroxybutryate, which contributes to the acidosis seen in DKA, are generally absent or are present in the plasma in negligible quantities (Scott & Claydon, 2012).
3.2 DKA
DKA arises in the absence of insulin, that leads to a combination of imbalances in other hormones that control glucose metabolism. Levels of glucagon, catecholamines, growth hormones and cortisol increase which, in turn, causes a decrease in glucose uptake and metabolism, and an increase in extracellular glucose leading to hyperglycaemia (Barrett, 2010; Kitabchi et al., 2009). These hormones also stimulate lipolysis to generate an alternative energy source in the absence of glucose uptake by cells. This leads to the liberation of fatty acids that are oxidised to ketones, most significantly β-hydroxyburyrate which contributes to‘diabetic ketoacidosis’ (DKA) causing coma, if not treated (Nazarko, 2009; Misra, & Oliver 2015.
3.3 A summary of the key differences in the symptoms of DKA and HHS.
Table 1 lists the key differences in the symptoms demonstrated by patients suffering from DAK and HHS. It is important to note, however, that these may not be mutually exclusive and the clinical presentation may be complicated by the existence of both conditions, as noted by the JBDS (Scott and Claydon, 2012).
|
Table 1: Differences in symptoms and simple diagnostics demonstrated by HHS and DKA (reproduced from Kisiel & Marsons, (2009) unless specified otherwise. |
|
DKA
|
|
HHS
|
Although these initial features of HHS and DKA may be evident on the patient’s admission to the ED, the addition of a full laboratory biochemical profile of blood plasma is normally conducted to aid a more specific diagnosis. The biochemical differences between the two conditions are summarised in Table 2. As is evident from this information, there are varied opinions regarding diagnostic biochemical indicators for HHS and DKA. This illustrates the lack of consensus on the diagnostic criteria evident in much of the literature relating to these two diabetic crises, that will be reviewed in chapters 4 and 5. Please note that Kitabchi’s (2009) review refers to American Diabetic Association (ADA) guidelines, and Scott and Claydon (2012) refers to the UK and US collaborative JBDS guidelines for the management of HHS (see Appendix 4).
|
Table 2: The Biochemical profiles of HHS and DKA measured in blood plasma. (reproduced from Kisiel & Marsons, 2009 unless specified otherwise) |
|
DKA
|
|
HHS
|
Typical deficits in water and electrolytes are reported by Nyenwe and Kitabchi (2011) and summarised in Table 3.
|
Table 3: Typical water and electrolyte deficits in DKA and HHS (cited Nyenwe and Kitabchi (2011) |
|
DKA
|
|
HHS
|
Although the symptoms and biochemical profiles of both DKA and HHS have been presented to support understanding of discussion of HHS treatment protocols and their crucial differences to those DKA, the following chapters focus upon the evidence based treatment of HHS. Although a precise set of diagnostic criteria does not exist, according to the JBDS (Scott & Claydon, 2012), the characteristic features that distinguishes HHS from DKA are hypovolemia, high level of plasma glucose (30 mmol/L or more) (which is accompanied by no ketones or ketones at a concentration of < 3 mmol/L) and an osmolality of 320 mOSm/Kg or more.
Chapter 4: Literature review methodology
4.0. Overview
Before a description of the methods used to formulate a specific research question that will be used to guide the search strategy, and the search strategy methodology, it is important to revisit the relevant objective that these processes aim to fulfil, which is ‘To inform nurses of the most recent, evidence and validated treatment protocols for the safe management of HHS’. In the absence of NICE guidelines to direct HHS management and diagnosis, the JBDS protocol (Scott and Claydon, 2012), (Appendix 4) is recognised by the NHS.
However, fieldwork research by this author revealed that other protocols for HHS treatment and opinions exist (see Table 3), many NHS trusts use their own protocols and, since the publication of the JBDS guidelines by Scott and Claydon (2012), research has continued in this area generating findings that could, potentially, impact upon them.
4.1. The formulation of a specific research question
This was achieved using the People, Exposure, Outcome (PEO) framework, summarised in Table 4 (adapted from Bettany Saltikov, 2012:21)
|
Table 4: Formulation of a specific research question using PEO framework |
||
|
People |
Exposure |
Outcome |
|
Adults admitted to emergency departments worldwide suffering from HHS |
The JBDS guidelines for diagnosis and management of HHS in adults (Scott and Claydon, 2012) or Contemporary treatment protocols or interventions advocated by published literature |
Collation of the most recent*, validated evidence to support interventions applied in the diagnosis and management of HHS |
*Most recent is defined as: Published subsequent to the JBDS (Scott and Claydon, 2012) guidelines, hereafter referred to as the ‘2012 JBDS HHS care pathway’)
The research question derived from PEO analysis was thus:
What are, currently, the safest, and most effective evidence based interventions available to inform clinical decision making in the management and diagnosis of HHS in adults?
4.2. The search strategy.
The search strategy was derived from the research question using Facet analysis. Facet analysis breaks topics down into components through the transposition their components into descriptive terminology (Spiteri, 1998). Facet analysis, based upon the PEO framework applied to generate the research question, was used to define search terms (see Table 5) and guide the overall search. The table shows the CINAHL research results, as an example.
|
Table 5: The search plan and FACET analysis |
||
|
Facet Analysis |
||
|
P |
E |
O* |
|
HHS Hyperosmolar hyperglycaemic state/syndrome/crisis/emergency Hyperglycaemic crisis Hyperglycaemic coma Hyperglycaemic emergency |
2012 JBDS guidelines ‘HHS management in adults’ (Scott and Claydon, 2012) |
Other protocols, recommendations, guidelines for HHS management published since 2013 Interventions for HHS management |
|
Search terms |
||
|
HHS OR Hyperosmolar hyperglycaemic state OR Hyperosmolar hyperglycaemic syndrome OR Hyperosmolar hyperglycaemic crisis OR Hyperosmolar hyperglycaemic coma OR Hyperglycaemic crisis OR Hyperglycaemic coma OR Hyperglycaemic emergency |
N/A |
Recommendations OR Findings OR Guidelines OR Protocols OR Management OR Treatment OR Outcomes |
|
No. of Hits (search P) = 31 |
No. of hits search (E) =1 |
Number of its searches (O) = 349, 219 |
|
Combine (P) and (O) using AND to give final number of hits = 31 |
||
|
Limits applied to all searches: English language, studies in adults, peer reviewed, references available, published since August 2013 |
||
|
Exclusion criteria for final study selection: Studies relating to paediatric HHS, DKA, published before August 2013. |
||
Three large healthcare databases were searched applying this strategy illustrated above. These were Medline (National Institute of Health, (NIH), 2016), the Cumulative Index of Nursing and Allied Health Literature (CINAHL) (Ebsco Health, 2016) and EMbase, (Elselvier R&D Solutions, 2016). Searching more than one database (ideally at least three) improves retrieval efficiency and minimises bias that can arise by failing to account for all relevant studies (Polit & Beck, 2010; Parahoo, 2014; Bettany Saltikov 2012)
Medline is an excellent source of quality primary research, and indexes a large proportion of clinically related Randomised Controlled Trials (RCT’s) that underpin bedrock of evidence based practice (Grove et al., 2014). CINAHL was selected because it includes literature directly relating to nursing practice. Embase was selected because behavioural psychology literature comprises 14% of the database, and, based upon the authors fieldwork, HHS may be precipitated by behaviours such as poor adherence to type 2 DM management regimes (Elliott et al., 2014).
If you need assistance with writing your dissertation, our professional Dissertation Writing Service is here to help!
Find out moreTo replace the authors dependence upon the presence of keywords in the abstract or title and enable whole articles to be searched Medical Subject Headings (MeSH’s) MeSH’s (also available in EMbase, and the equivalent in CINAHL), were browsed in addition to inputting the search terms as key words. The ‘browsing’ facility ensures the inclusion of all related terms and alternative spellings. For example, studies referring to the words ‘hyperglycaemic’ and ‘hyperglycemic’ will be retrieved. Search terms were broadened using MeSH’s ‘explode’ (‘exp’) function. This reduced the risk of failing to retrieve related studies that may have useful information but were not specified in the search strategy- it provided a ‘safety net’ in other words.
As indicated in Table 5, Boolean operators ‘AND’ and ‘OR’ were applied to direct the search towards retrieval of the most relevant publications. OR was used to retrieve as many studies as possible and (in addition to ‘expand’ outlined below) reduced the risk of missing informative articles, but retrieved a large number of articles that were not sufficiently specific to the research question. This was overcome by using the ‘AND’ function which acts as a filter, which connected the search terms and retrieved only citations that are relevant when all expanded search terms were combined.
Where necessary, truncation was applied (see Table 7). This feature of Medline, CINAHL and Embase, in addition to the ‘expand’ function saves the researcher from having to include all spellings, plurals, or alternative endings to search terms.
|
Table 7: Truncation functions |
|
|
* Or $ |
Retrieves the stem word along with all possible endings: hyperglycem*= hyperglycemic, or hyperglycemia |
|
# |
Replaces a character: wom#n= hyperglyc or women |
|
? |
Replaces one character or none: Hyperglyc?emic will retrieve hyperglycaemic or hyperglycemic. c |
4.3. Search Inclusion and Exclusion Criteria
The search strategy included all peer reviewed journal articles published over the past 5 years, i.e. subsequent to the 2012 JBDS HHS care pathway. This limited the search for literature from (and including) August 2012 to the present day. There is no facility to obtain articles that have yet to be indexed from these databases, therefore, any searches currently undergoing this process risk missing very recent studies.
Exclusion criteria applied included all studies up to August 2012, because the 2012 JBDS HHS care pathway incorporates all relevant, validated, and evidence based studies up to that date. Non-English articles were excluded because these could not be comprehended by the author, additionally studies based on children and on adults suffering exclusively from DKA were excluded because the findings of these publications would not be relevant to the research question. All non-journal articles, for example books, editorials, opinion pieces and letters were excluded on grounds of lack of validation by peer review and low generalizability of their findings, in addition to single case studies, which were excluded for the same reason.
The search results from all three databases provided in chapter 5.
Chapter 5: Results of the literature search and critique of selected articles.
5.0. The search outcome
The search results, i.e. the number of articles (‘hits’) retrieved by applying the search strategy to all three databases is summarised in Table 8. The selection process is presented by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram, advocated by Moher et al., (2009), in Appendix 1.
The abstracts, and if necessary, full text of all retrieved articles were scrutinised for evidence based recommendations or findings relevant to HHS diagnosis and management.
|
Table 8: summary of initial search yield for each database |
||
|
Database |
Number of hits |
Relevant citations retrieved |
|
Medline |
49 |
38 (11 excluded articles related to DKA or unrelated conditions) |
|
Embase |
As above |
As above |
|
CINAHL |
31 |
29 (4 excluded because related to DKA or unrelated conditions) |
|
Number of relevant articles cited by all three databases= 109. After duplicates removed = 38 |
||
The selected texts are asterisked in the reference list, where full bibliographical details are provided. The articles selected include two articles presenting protocols for the management of diabetic hyperosmolar hyperglycaemia, and eight reviews. The search retrieved several articles that discussed the management of HHS arising from other conditions or exposure to pharmaceutical drugs (iatrogenic causes). These were discarded on the grounds of relevance to the research question, as were articles that focused only upon the aetiology and epidemiology of HHS. All articles rejected by this process, with rationales for their rejection, are cited in Table 2A, Appendix 2 to provide transparency of the review and to prevent reviewer bias (Shea at al., 2007).
The recommendations made by the 10 selected articles were synthesised and are summarised in the data extraction table (Table 3A, Appendix 3). The ‘benchmark’ comparator applied is the 2012 JBDS HHS care pathway (Scot and Claydon, 2012 pp. 25-30), reproduced in Appendix 4. Recommendations were categorised into specific interventions in HHS management, as provided by the JBDS HHS care pathway.
Before the information comprising the recommendations and findings of the articles can be considered in relation to nursing practice, it is important to evaluate the quality of the selected articles. Haber and Lo-Biondo Wood (2014) emphasise the critical appraisal of research is imperative in order to determine objectively, and without bias, the validity of research findings, their generalizability and the reliability with which they can be safely translated into practice, to achieve the best possible patient care.
There are various tools available to guide the critique process (examples can be found in Craig and Smyth, 2002). Typically, these apply a ‘checklist’ to guide the reviewer’s evaluation. The application of validated tools such as the Critical Skills Appraisal Programme (CASP) checklists is important to prevent reviewer bias and ensure that the evaluation process is thorough. A CASP (2017) review specific checklist was selected to guide the critical appraisal of the eight selected reviews. This framework was applied because CASP has been widely validated for its efficacy in appraising health related research (Ciliska et al., 2010; NICE (2016b). Furthermore, the review specific checklist has been tested by health care professionals and is based upon an established review critique format initially developed by Oxman et al., (1994). Part 5.1 below provides a summary of the critical appraisal of all eight reviews in which the most salient points are raised.
5.1 Critical appraisal of selected articles
Initially, the CASP (2017) checklist prompts the reviewer to consider if the reviews identify and address a focused question and if the authors referenced appropriate sources (in this instance, this should, ideally, be primary research published in peer reviewed journals). This is because, it can be argued, a review is only as good as the research it draws upon, a principle applied to systematic reviews highlighted by Bettany-Saltikov (2012) when discussing the hierarchy of research evidence. CASP (2017) suggests excluding studies in which these criteria are not met from further review. In this instance, all eight reviews identified a focused question (although not all with complete clarity; Maletkovic and Drexler (2015) do not immediately get to the point regarding the rationale for their review. Although appropriate sources were generally cited, there were exceptions, in which sources were either out of date or not peer reviewed. For example, McCombs et al (2015) reference Kitabchi et al., (2014) when citing the criteria for change in fluid regime from 0.9% to 0.45% Sodium Chloride. This reference article has not been published in a peer reviewed journal; rather it is an online source available to personal subscribers to ‘UptoDate.com’.
Out of date referencing is evidenced by Gouveia and Chowdhury, (2013) who cite Scott (2006), ignoring the recently published Scott and Claydon (2012) JBDS guidelines that supersede Scott’s (2006) publication, regarding the management of HHS. Maletovic and Drexler (2013) do not reference or mention Scott (2006) nor Scott and Claydon (2012), despite these publications providing what would have been, (at the time of Maletovic and Drexler ‘s writing) amongst the most detailed, evidence based studies available.
The omission of important references to key points and recommendations appears to be common across all eight reviews, including Scott’s (2015) review of the JBDS HHS management recommendations (Scott and Claydon, 2015). For example, an extremely important aspect of HHS management is the administration of supplementary insulin. The JBDS guidelines (Scott and Claydon, 2012), reiterated by Scott (2015), state explicitly that insulin should only be commenced when treating HHS if there is significant ketoanemia (indicating that the patient’s condition is partly caused by DKA). According to Scott (2015), Scott and Claydon (2012), Dhatariya (2014) and Gouveia and Chowdhury (2013), the absence of ketones indicates the presence of residual insulin production and supplementation is not only unnecessary, but could lead to hypoglycaemia and a fluid shift from the extracellular to intracellular fluid, precipitating cardiovascular collapse; yet this crucial point is not specifically referenced in any of these publications. Therefore, how can health care practitioners regard these recommendations as reliable, let alone evidence based?
Similarly, the recommendations made by all reviews to change fluid infusion from 0.9% sodium chloride solution to 0.45%, depending upon cited criteria, is not consensual. For example, 2012 JBDS HHS care pathway, Scott (2015) and Gouveia and Chowdhury (2013) recommend making this change if serum osmolality is not falling despite the achievement of positive fluid balance, but offer no evidence based, referenced rationale. Van Ness-Otunnu cites the ‘ADA guidelines’ (without a reference) that state the criteria as hypernatraemia or normal sodium levels, which is also recommended by Corwell et al. (2014), again, without referencing any primary source of evidence. Frustratingly, the only review that does cite a reference to support this recommendation is McCombs at al. (2015), who cites Kitabchi, 2014, but, as discussed above, this is not peer reviewed.
In addition to the failure by all eight reviews to reference significant recommendations, exemplified by the studies discussed above, there is little evidence that authors have assessed the quality of the studies or research included for review. They may well have done so, and been satisfied, but this is not made explicit in any of the reviews. Arguably, this is more a requirement of a systematic review, which none of the reviews under evaluation here claim to be. Nevertheless, in the absence of any systematic reviews or meta-analyses of interventions applied to manage HHS, some indication of the quality of research, where cited, would, be informative and confer credibility.
A common problem arises from many authors’ failure to reference primary sources of information pertinent HHS management. This is exemplified by the popular citing of articles by Kitabchi, 2001, 2006, 2008 and 2009, at least one of which is cited by all of the reviews selected (1-8 in Table 1A, Appendix 2), by both of the protocols (9-10 in Table 3A, Appendix 3) and by the current 2012 JBDS HHS care pathway. Kitabchi et al’s (2001), (2008) and (2009) articles are reviews that extensively reference primary research literature, and a small number of other reviews. Kitabchi et al.’s (2006) article is a consensus statement of the American Diabetes Association hyperglycaemic crises guidelines, and is, essentially, a review that again refences extensive primary research.
The problem with citing a reference, in a review, without making it clear to the reader that the source is another review, is that the review itself becomes a tertiary source of the information concerned. This raises issues relating to the quality and reliability of the information, which is a significant concern if there is an intention to apply it to clinical practice. Crucially, without access to information about the primary research, the reader cannot judge its quality, reliability, the influence of any bias from researchers or the authors of the review nor gain any indication of the transferability or generalizability of the information to their own practice.
Furthermore, it is difficult to see how a review can offer any new information or make any real contribution to the implementation of evidence based practice if all it does is simply relay previously published information and offers no new perspective. For example, Gouveia and Chowdhury (2013) cite Kitabchi et al’s (2009) review in stating that DKA has a more acute presentation (typically < 24 h) than HHS. Kitabchi, et al (2009) references this assertion to the Kitabchi et al (2001) review. The latter not only reveals that the 2009 review precisely reproduces the same paragraph, but provides no reference at all to support this information. Therefore, how true is this DKA has a presentation that is typically <24 hours, based upon this reference? There is no research evidence available to make a judgement. This is an isolated example, yet the reviews selected demonstrate many similar assertions, particularly when referencing Kitabchi at al.’s work. In many respects, therefore, review’s such as Gouveia and Chowdhury (2013) merely pass information along without ascertaining its accuracy or bias in its reporting. The practice, which is by no means isolated to Guoveia and Chowdhury’s article can be likened to passing a rather old ball along, in Rugby, without looking at it.
Overall, the reviews provide a useful theoretical background to health professionals encountering patients admitted to emergency departments with HHS. Scott (2015), Gouveia and Chowdhury (2013) and Dhatariya (2014) provide a clear summary and reiteration of the 2012 JBDS HHS care pathway whereas Corwell et al., (2014) summarises the Canadian Diabetic Association (CDA) guidelines and Maletkovic and Drexler (2013), together with McCombs et al (2015), provide an American perspective. Collectively the reviews illustrate slight variations in fluid resuscitation regimes, some significant differences in the timing of insulin administration, a general consensus in the maintenance of plasma potassium and the lack of need for bicarbonate supplementation. Bicarbonate is normally only warranted in cases of severe acidosis, which indicates DKA or a pronounced DKA component to HHS. Lenahan and Holloway (2015) and other reviews that address bicarbonate supplementation in any detail, such as Corwell et al., (2014), do so in relation to DKA alone, and recommend bicarbonate replacement only if blood pH falls below 6.9.
Two protocols published subsequent to the 2012 JBDS HHS care pathway were retrieved from the literature search, in addition to the reviews discussed above. As previously emphasised, reaching an unbiased objective judgement regarding the protocols’ transferability to healthcare practice requires the application of a validated appraisal tool. In the absence of a CASP critical appraisal checklist for clinical protocols or guidelines, a comparable, widely applied, American appraisal system was selected. The Advancing Guideline Development Reporting and Evaluation in Healthcare (AGREE II) checklist (Brouwers et al., 2010a, 2010b and 2010c) has been used to guide the evaluation outlined below, and key points from this are discussed below.
The two protocols (Goguen and Gilbert., 2013) and Miyares et al., (2013) are both five years old and comprise the only published clinical guidelines for the treatment of HHS published since the 2012 JBDS HHS care pathway (Scott and Claydon, 2012). Both protocols clearly outline their objectives, Goguen and Gilbert., (2013) (hereafter referred to as ‘Goguen and Gilbert’) state that the treatment guidelines are targeted at an adult population, Miyares et al. (2013 (hereafter referred to as Miyares et al.) do not specify a target population. Therefore, although the purpose of the guidelines is specified by both authors, only Goguen and Gilbert outline the scope of the CDA guidelines presented. Although both protocols outline the health questions addressed by their protocol, these are presented in the accompanying text rather than stated explicitly with reference to the guidelines suggested.
Stakeholder involvement is presented clearly by Miyares et al., at the end of the article and all necessary details such as affiliation. Involved individuals’ roles in protocol development and their location are provided. This information is not provided by Goguen and Gilbert, but this may be because the information has been presented elsewhere. Nevertheless, if this is the case, stakeholder information should be available via a referenced source. Similarly Goguen and Gilbert provide no information whatsoever regarding the development of their protocol, literature searched or any indication of how information was collated when designing the protocol, nor is this referenced to an additional source. In addition, the target users of the protocol are not identified (for example, nurses and/or physicians), nor is there any indication of how the guidelines should be used. This is in contrast to Miyares et al., who provide a detailed description of protocol development, outline how the views of stakeholders were captured and applied in the process, state nurses as the protocols targeted users and clearly present a format for its application.
The literature search conducted by Miyares is summarised but not in sufficient detail for it to be reproduced (for example, search terms, medical subheadings and a search strategy are not identified), nor are the databases searched listed.
Miyares et al., make some reference to the strengths of the evidence they have used, for example they refer to applying randomised controlled trial data when discussing their recommended insulin administration route, and to a systematic review when justifying their bicarbonate replacement strategy. Although no weaknesses in the studies referenced is indicated, this may reflect the authors’ selection of only high quality research (such as systematic reviews). Goguen and Gilbert present an appropriately referenced literature review (within their text accompanying the protocol) but this cannot be assessed for bias or thoroughness because no search strategy is evident.
The strengths or weaknesses of the studies referenced are not indicated nor is the development of the protocol discussed or referenced. Overall, the strengths and weaknesses of Goguen and Gilbert’s protocol are not addressed by the authors nor are any references made to an evaluation process that could support the validity of their recommendations. Furthermore, although a recent CDA publication (Alkhiari et al., 2017) sought to evaluate adherence to, and the efficacy, of guidelines for the management of in-hospital type 2 patients in preventing hyperglycaemia, these authors make no reference to the hyperglyaemia treatment guidelines, probably because none of the patients selected as part of the appraisal were metabolically compromised. Therefore, the most recent CDA guidelines for the treatment of hyperglycaemia published by Goguen and Gilbert have yet to receive any clinical validation.
Miyares et al describe the development of their protocol is some detail, explain pilot testing, modifications made based upon stakeholder feedback and the committee appointed to oversee the production of the guidelines. Although limitations are not made explicit, future quality improvement plans are provided. To date, no further studies have been published that report upon the extent of application of Miyares et al.’s protocol, nor its efficacy in safely improving patient outcomes, therefore, in common with Goguen and Gilbert’s CDA guidelines, it remains unvalidated.
There is, however, a general lack of quality assurance studies conducted that enable the safety and efficacy of guidelines to be evaluated, and which provide some indication of the reliability and validity of suggested protocols. This applies particularly to the in-hospital management of diabetes and its complications (Alkhiari et. al., 2017). The limited number of studies that have examined adherence to hyperglycaemia treatment protocols have focused upon DKA, and report low adherence rates, largely caused by practical difficulties and lack of coordination amongst the multidisciplinary team (Devalia, 2011; Salahuddin & Anwar, 2009, and Fusco et al., 2015).
This deficit in evaluation extends to the 2012 JBDS HHS care pathway which, although compiled by a multidisciplinary team and piloted before publication, have yet to be evaluated for the extent of their application and adherence by practitioners and/or hospital Trusts, nor their safety and efficacy in improving patient outcomes.
Chapter 6: Discussion: implications for practice.
Before considering the implications of the information presented by the eight reviews and two protocols, it is helpful to revisit the research question:
What are, currently, the safest, and most effective evidence based interventions available to inform clinical decision making in the management and diagnosis of HHS in adults?
As discussed above, the overall quality of the reviews selected, which comprise those most recent and relevant to the research question, is poor. It is, therefore, unsurprising that the data extraction revealed many contradictions and vague statements within the recommendations for HHS management. One of the major criticisms offered in the previous chapter was the reproduction of previously published information, with frequent citations of reviews, or even reviews of reviews. The British reviews, (Scott, 2015, Dhatariya, 2014, and Gouveia and Chowdhury (2013) simply summarise the 2012 JBDS HHS care pathway. No additional information or research based evidence is offered by any of these studies.
However, this criticism is only valid if there has been significant research regarding the management of HHS in adults conducted since the 2012 JBDS HHS care pathway was published. To date, there have been no systematic reviews or meta-analyses published that address any aspect of HHS management in adults. Several non-generalizable case studies have been published (listed in Table 2A, Appendix 4), many of which discuss conditions secondary to HHS, and in terms of aetiology as opposed to treatment. Therefore, at the time all of the reviews included in this dissertation were written, there were no new, significant studies available for the authors to include. This is a situation that endures to the present day, exemplified by the results of the authors literature search which retrieved reviews, rather than primary research studies specifically addressing HHS treatment interventions. This probably reflects the small HHS incidence rate, compared to that of DKA, discussed in chapter 1. This poses practical problems in recruiting sample populations large enough to generate data amenable to rigorous statistical analysis. Such an approach would require a large, multi-centre study, probably extending across several countries.
If you need assistance with writing your dissertation, our professional Dissertation Writing Service is here to help!
Find out moreAll of the remaining reviews (listed in Table 3A, Appendix 3) are American, and none make any reference to the 2012 JBDS HHS care pathway. Instead, alternative recommendations are suggested, based upon the equivalent America Diabetic Association (ADA) guidelines (reproduced in Kitabchi’s 2009 review). For example, fluid resuscitation that is titrated to osmolality is advocated by the 2012 JBDS HHS care pathway, whereas the ADA recommend stated rates (for example 1L/h) with further rates adjusted depending upon urine output, haemodynamics and laboratory investigations. A second key difference is that the ADA recommend initiating low dose IV insulin before dehydration has been corrected. This is explicitly contradicted by the JBDS, which recommends this action only if there is significant ketoneamia (defined by 3β-hydroxy butyrate > 1mmol/L).
Maletkovic and Drexler (2013) provide one of the better American reviews in terms of detail, accuracy of referencing and clarity. Their initial assessment criteria is particularly useful given that neither of the two protocols retrieved (articles 9 and 10 on Table 3A, Appendix 3) provide any assessment criteria. The 2012 JBDS HHS care pathway does so, but does not suggest such detailed clinical investigations. This may reflect funding differences between American and British healthcare systems; for instance, chest X rays are suggested to ascertain the cause of HHS. This is not suggested by the JBDS, presumably on the grounds of cost: a chest X ray costs a typical NHS trust (such as Stockport) £25 (Sillitoe, 2014). Nevertheless, it perhaps worth referring to Maletkovic and Drexler’s (2013) assessment criteria in situations in which obtaining a diagnosis proves problematic, despite the application of JBDS HHS assessment criteria.
Lenahan and Holloway (2015) provide some useful nursing insights, from an American perspective, highlighting the higher risk faced by type 2 diabetic patients of Hispanic and African origin, and emphasising the importance of regular or continuous monitoring, particularly to prevent hypokalaemia, which poses significant risk of death in patients experiencing a hyperglycaemic crisis (Morales & Rosenbloom, 2004).
The two protocols reviewed include the CDA recommended guidelines for the management of HHS in adults (Goguen and Gilbert, 2013) and a local protocol designed to be applied within Jackson Memorial Hospital, in the USA (Miyares et al., 2013). These protocols have already been subject to critique, and compared in Table 1A (Appendix 3) to the 2012 JBDS HHS care pathway. The question is, are they any better? None have been validated, nor have their efficacy and safety in improving outcomes for HHS patients been evaluated. It is not even known how well the protocols have been adhered to at local level, since their development.
Therefore, it is difficult to make a judgement of which are ‘currently, the safest, and most effective evidence based interventions available to inform clinical decision making in the management and diagnosis of HHS in adults’.
In terms of relevance to nursing practice, it is the author’s opinion that the 2012 JBDS HHS care pathway supported by Scott’s (2015) review, fulfil the research question most reliably. The advantages of the 2012 JBDS HHS care pathway in relation to nursing practice, not evident in the other guidelines, include the emphasis placed upon titrating fluid infusion rates to serum osmolality, prompting appropriate action should patients become hypokalaemic or hypoglycaemic, and the attention paid to pressure sore prevention. Preventing pressure sores is an extremely important element of the nursing care of patients who are in a comatose state, even more so if they are diabetic and at significantly higher risk than non- diabetic patients of developing foot ulcers, (reviewed by Hill, 2009).
Although aftercare is not a topic of this dissertation, it is important that nurses caring for patients being treated for HHS are aware of the potential future psychosocial impact of the admission, which will generally be to a high dependency area to provide continuous monitoring.Much has been written about the long term psychological effects of admission to high dependency or intensive care units, that include post-traumatic stress disorder, and the effects of sedation, that are beyond the scope of this study. However, useful reviews are provided by Davydow et al., (2008), Griffiths et al., (2007) and Jackson et al., 2007.
The psychological effects upon the patient and their family of this experience may be even more acute if the diabetes had not been diagnosed and HHS represents a presenting symptom. Hackel, (2014), a nurse practitioner, describes treating such a patient and describes having to overcome the patient’s initial ‘scepticism’ at his diagnosis in order for the him to become receptive to teaching. In this example, Hackel used empowerment techniques to improve the patient’s confidence in self-care and decision making about his futures diabetes management. Patients who have experienced HHS and been treated in hospital according Scott and Claydon’s (2012) JBDS HHS management protocol will have received intensive interventions to correct their fluid balance over a 72 hour period, and will thus have experienced an episode of incapacity. Some period of rehabilitation and impact upon their quality of life is inevitable and nurses are ideally positioned in this respect to ensure referrals to outside agencies such as social services are made, if necessary. Regardless of the patient’s previous history of diabetes, providing education to prevent such an episode reoccurring is imperative, and recommended by the JBDS HHS guidelines as part of aftercare delivered when the immediate crisis is resolved.
In patients with a previous diagnosis of type 2 DM, precipitating factors of HHS include poor adherence to treatment regimes, poor dietary control or a pathological underlying cause, such as infection (Kitabchi,et al., 2009). Although Elliott et al, (2014) demonstrated the value of a structured training approach to insulin therapy in reducing the incidence of DKA in type 1 DM, no comparable studies have been conducted to evaluate structured approaches that could prevent reoccurrence of HHS in type 2 DM patients.
However, a large American randomised controlled trial (Gregg et al., 2012) has examined the efficacy of intensive lifestyle interventions in achieving remission of type 2 DM, which reduces the risk of associated complications including hyperglycaemic crisis. Interventions focused on weight loss as an identified, modifiable risk factor and, combined with education and support, increased the likelihood of some remission, with a modest increase in absolute partial remission rates.
Therefore, Identifying the underlying cause of HHS to determine the influence of modifiable factors that are amenable to prevention or modification may reduce the risk of reoccurrence. This could include providing dietary and exercise advice, supporting adherence to treatment regimens and raising awareness of potential trigger factors such as infection. The role of nurses caring for HHS patients in the recovery phase demands close liaison with the multidisciplinary team, ensuring appropriate referrals are made for the patient post discharge. It is particularly important that GP’s and specialist nurse practitioners are aware of particular risk factors that could contribute to HHS affecting individual patients, such as ethnicity (highlighted by Maletkovic and Drexler 2013) in order to respond by adapting their monitoring of patients’ diabetes control, and adherence to therapy, appropriately. In addition, there is some evidence (Wang et al., 2014) that HHS may increase the risk of ischaemic stroke, the prevention of which requires additional vigilance to maintaining optimal cardiovascular health by the patient and their primary care team.
Chapter 7: Summary and conclusions
This dissertation has identified, synthesised and critiqued recent literature relevant to the research question.
What are, currently, the safest, and most effective evidence based interventions available to inform clinical decision making in the management and diagnosis of HHS in adults?
In doing so, it has been possible to consolidate currently accepted treatment recommendations from British and American and Canadian perspectives, highlighting their differences. The 2012 JBDS HHS care pathway remains, in the authors opinion, those most likely to safeguard the wellbeing of patients with HHS, which is a professional requirement of nursing care demanded by the NMC (2015).
The reasons are that, by titrating fluid infusion rates with serum osmolality, the dangers associated with too rapid fluid replacement, which include cerebral oedema or central pontine myelinolysis, can be avoided. No other protocols make this recommendation. The 2012 JBDS HHS care pathway prompts senior medical review in instances where potassium has fallen to a defined limit (< 3.5 mmol/L), thereby safeguarding against hypokalaemia, which again is absent in the other two protocols reviewed. The 2012 JBDS HHS care pathway guidelines include several prompts to seek senior advice if the patient is not improving despite application of the recommended interventions, suggest when to refer to the multidisciplinary team and ensure preventative measures to prevent foot ulceration are in place. Again, these patient safety measures are not reflected by the other two protocols, published subsequent to the 2012 JBDS HHS care pathway.
Returning to the objectives of the dissertation:
1) To gain awareness of the most recent, evidence based treatment protocols and recommendations made for the treatment of HHS.
These have been summarised in the data extraction table 1A, Appendix 2, are listed in the reference list and have been critiqued, with the most important points arising summarised in chapter 3.
2) To compare the above information with the recommendations made by the JBDS (Scott and Claydon, 2012), which apply in the UK in the absence of NICE guidelines relating to this condition.
Table 1, Appendix 2 provides this comparison, which is developed further in chapter 6.
3) To gain sufficient evidence based knowledge to question and treatment of HHS that appears inappropriate.
In addition to the information presented in the text, a useful review (Scott, 2015) which supplements the Scott and Claydon’s (2012) JBDS HHS care pathway, has been identified as providing the most useful evidence based information for nurses when caring for HHS patients, and therefore supports nurses’ fulfilment of the NMC (2015) requirements to deliver evidence based practice, as far as possible.
This study has strengths and weaknesses. Importantly, the dissertation encourages readers not to take the recommendations presented in review articles or protocols at face value, but to apply the same level of critical appraisal to them as they would primary research, if the intention is to apply findings to practice. Two appropriate tools in which to achieve this have been identified, and applied. Furthermore, the critique offered in chapter 3 exemplifies the degree of attention that must be paid to references cited by authors of reviews, in order to determine their reliability. An example is provided in chapter 3 demonstrating how unreliable information can be perpetuated by non-critical appraisal and poor referencing.
The search strategy of three databases was extremely thorough, reported transparently in chapter 2 (enabling its reproduction), and with non-selected studies listed in Appendix 3, with a rationale for their exclusion. Therefore, the author has minimised reporting bias as far as possible. A shortcoming of the search strategy was that it retrieved a large number of case studies, and studies that referred to the epidemiology and aetiology of HHS, many associating the condition with other comorbidities. Although an alternative search strategy could have removed these, the author balanced this against the risk of missing important pieces of research and did not adjust the strategy.
The scope of the dissertation could have been wider, and included paediatric HHS management. However, this would not have offered any insights in terms of treatment interventions relevant to the 2012 JBDS HHS care pathway, which refers only to HHS management in adults.
References
Abate, K.S. and Buttaro, T.M., 2015. Safe and effective NSAID use. The Nurse Practitioner, 40(6), pp.18-22.
Alkhiari, R., Alzayer, H., Aljazeeri, J., Vanniyasingam, T. and Punthakee, Z., 2017. Adherence to Guidelines for Inpatient Pharmacologic Management of Type 2 Diabetes and Glycemic Outcomes. Canadian Journal of Diabetes. Pp1-5 (note to QC- no volume/issue assigned yet by the journal)
Almdal T, Scharling H, Jensen JS, Vestergaard H. 2004 The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke and death: a population-based study of 13 000 men and women with 20 years of follow-up. Arch Intern Med 164: pp.1422–1426
Alva, M.L., Gray, A., Mihaylova, B., Leal, J. and Holman, R.R., 2015. The impact of diabetes‐related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabetic Medicine, 32(4), pp.459-466.
Andersen, M.K., Pedersen, C.E.T., Moltke, I., Hansen, T., Albrechtsen, A. and Grarup, N., 2016. Genetics of type 2 diabetes: the power of isolated populations. Current diabetes reports, 16(7), p.65.
Astrup, A., 2001. Healthy lifestyles in Europe: prevention of obesity and type II diabetes by diet and physical activity. Public health nutrition, 4(2b), pp.499-515.
Atkinson, M.A., Eisenbarth, G.S. and Michels, A.W., 2014. Type 1 diabetes. The Lancet, 383(9911), pp.69-82.
Barrett, K.E., 2010. Ganong's review of medical physiology. McGraw-Hill Education, Maidenhead (UK).
Bettany-Saltikov, J., 2012. How to do a systematic literature review in nursing: a step-by-step guide. McGraw-Hill Education. Maidenhead, (UK).
Blouin, D., 2012. Too much of a good thing. Canadian Family Physician, 58(10), pp.1099-1100.
Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 2010; 464: pp1293–300.
Brouwers M, Kho ME, Browman GP, Cluzeau F, feder G, Fervers B, Hanna S, Makarski J (on behalf of the AGREE Next Steps Consortium) 2010a. AGREE II: Advancing guideline development, reporting and evaluation in healthcare. Can Med Assoc J. 182:E839-842; doi: 10.1503/cmaj.090449.
Brouwers M, Kho ME, Browman GP, Burgers J, Cluzeau F, Feder G, Fevers B, Graham ID, Hanna SE, Makarski J, (on behalf of the AGREE Next Steps Consortium) 2010b. Performance, usefulness and areas for improvement: Development steps toward the AGREE II –Part 1. Can Med Assoc J. 182: 1045-52
Brouwers MC, Kho ME, Browman GP, Burgers J, Cluzeau F, Feder G, Fervers B, Graham ID, Hanna SE, Makarski J, on behalf of the AGREE Next Steps Consortium. Validity assessment of items and tools to support application: Development steps towards the AGREE II –Part 2. Can Med Assoc J. 2010, 182: E472-78
Canarie, M.F., Bogue, C.W., Banasiak, K.J., Weinzimer, S.A. and Tamborlane, W.V., 2007. Decompensated hyperglycemic hyperosmolarity without significant ketoacidosis in the adolescent and young adult population. Journal of Pediatric Endocrinology and Metabolism, 20(10), p.1115
Centers for Disease Control and Prevention, 2009. National Diabetes Fact Sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2008. Also available online at: http://www.cdc.gov Accessed 20 September 2017.
Chiasson, J.L., Aris-Jilwan, N., Bélanger, R., Bertrand, S., Beauregard, H., Ékoé, J.M., Fournier, H. and Havrankova, J., 2003. Diagnosis and treatment of diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Canadian Medical Association Journal, 168(7), pp.859-866.
Ciliska, D Thomas, H., Buffett, C. 2010. An Introduction to Evidence-Informed Public Health and A Compendium of Critical Appraisal Tools for Public Health Practice [online] Available at: http://www.nccmt.ca/uploads/media/media/0001/01/027d5748c39518c2e8b9c602fd0c4cb2860d3db0.pdf Accessed 20 September, 2017
Clancy, J. and McVicar, A., 2009. Physiology and Anatomy for Nurses and Healthcare Practitioners: A Homeostatic Approach. CRC Press. Boca Raton, Florida, US.
Concannon, P., Rich, S.S. and Nepom, G.T., 2009. Genetics of type 1A diabetes. New England Journal of Medicine, 360(16), pp.1646-1654.
*Corwell, B., Knight, B., Olivieri, L. and Willis, G.C., 2014. Current diagnosis and treatment of hyperglycemic emergencies. Emergency Medicine Clinics, 32(2), pp.437-452.
Craig, J.V. and Smyth, R.L. (Ed.’s) 2002.The Evidence-based Practice Manual for Nurses. Churchill Livingstone: Edinburgh. UK
Critical Appraisal Skills Programme Critical Appraisal Skills Programme (CASP), 2017. CASP Systematic Review Checklist. [online] Available at: http://www.casp-uk.net/casp-tools-checklists. Accessed 25 September 2017.
Davydow, D.S., Gifford, J.M., Desai, S.V., Needham, D.M. and Bienvenu, O.J., 2008. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. General hospital psychiatry, 30(5), pp.421-434.
De Beer, K., Michael, S., Thacker, M., Wynne, E., Pattni, C., Gomm, M., Ball, C., Walsh, D., Thomlinson, A. and Ullah, K., 2008. Diabetic ketoacidosis and hyperglycaemic hyperosmolar syndrome–clinical guidelines. Nursing in critical care, 13(1), pp.5-11.
Department of Health (DH), 2002. The Department of Health's response to the report of the Public Inquiry into children's heart surgery at the Bristol Royal Infirmary. [online] Available at: https://www.gov.uk/government/publications/the-department-of-healths-response-to-the-report-of-the-public-inquiry-into-childrens-heart-surgery-at-the-bristol-royal-infirmary Accessed 18 September 2017.
Department of Health, (DH) 2012. The NHS Constitution for England. [online] Available at: https://www.gov.uk/government/publications/the-nhs-constitution-for-england. Accessed 20 September 2017
Devalia, B., 2010. Adherence to protocol during the acute management of diabetic ketoacidosis: would specialist involvement lead to better outcomes? International journal of clinical practice, 64(11), pp.1580-1582.
*Dhatariya, K., 2014. Diabetic ketoacidosis and hyperosmolar crisis in adults. Medicine, 42(12), pp.723-726.
Diabetes UK, 2016. NHS Spending on diabetes ‘To Reach 16.9 billion by 2035 [online] Available at: https://www.diabetes.org.uk/About_us/News_Landing_Page/NHS-spending-on-diabetes-to-reach-169-billion-by-2035/ Accessed 28 September, 2017
Diabetes UK, 2017. Getting Active and Staying Active. [online] available at: https://www.diabetes.org.uk/guide-to-diabetes/managing-your-diabetes/exercise Accessed 17 September 2017
Dolan, C., Glynn, R. and Lawlor, B.A., 2017. A Delphi study to establish an expert consensus opinion on risk factors for type 2 diabetes, and potential complications of diabetes, including brain health associations. European Psychiatry, 41, p.S649.
Ebsco Health, 2016. CINAHL database [online] Available at: https://health.ebsco.com/products/the-cinahl-database Accessed 18 September, 2017
Eisenbarth GS. 2007. Update in type 1 diabetes. J Clin Endocrinol Metab. 92: 2403–7.
Elliott, J., Jacques, R.M., Kruger, J., Campbell, M.J., Amiel, S.A., Mansell, P., Speight, J., Brennan, A. and Heller, S.R., 2014. Substantial reductions in the number of diabetic ketoacidosis and severe hypoglycaemia episodes requiring emergency treatment lead to reduced costs after structured education in adults with type 1 diabetes. Diabetic medicine, 31(7), pp.847-853.
Elselvier R&D Solutions 2016 Embase fact Sheet [online] Available at: (https://www.elsevier.com/solutions/embase-biomedical-research Accessed 20 September, 2017
Fourtner, S.H., Weinzimer, S.A. and Levitt Katz, L.E., 2005. Hyperglycemic hyperosmolar non‐ketotic syndrome in children with type 2 diabetes. Pediatric diabetes, 6(3), pp.129-135.
Francis, R., 2013. Report of the Mid Staffordshire NHS Foundation Trust public inquiry: executive summary (Vol. 947). The Stationery Office. London UK.
Fusco, N., Gonzales, J. and Yeung, S.Y.A., 2015. Evaluation of the treatment of diabetic ketoacidosis in the medical intensive care unit. American Journal of Health-System Pharmacy, 72.
Gianani, R., Campbell-Thompson, M., Sarkar, S.A., Wasserfall, C., Pugliese, A., Solis, J.M., Kent, S.C., Hering, B.J., West, E., Steck, A. and Bonner-Weir, S., 2010. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia, 53(4), pp.690-698.
Gilis-Januszewska, A., Lindström, J., Tuomilehto, J., Piwońska-Solska, B., Topór-Mądry, R., Szybiński, Z., Peltonen, M., Schwarz, P.E., Windak, A. and Hubalewska-Dydejczyk, A., 2017. Sustained diabetes risk reduction after real life and primary health care setting implementation of the diabetes in Europe prevention using lifestyle, physical activity and nutritional intervention (DE-PLAN) project. BMC public health, 17(1), p.198.
Gill, G.V. and Alberti, K.G.M.M., 1985. Diabetic hypoglycemia. Practical Diabetes, 2(5), pp.5-10.
Godino, J.G., van Sluijs, E.M., Marteau, T.M., Sutton, S., Sharp, S.J. and Griffin, S.J., 2016. Lifestyle advice combined with personalized estimates of genetic or phenotypic risk of type 2 diabetes, and objectively measured physical activity: a randomized controlled trial. PLoS medicine, 13(11), p.e1002185.
* Goguen, J. and Gilbert, J., 2013. Hyperglycemic emergencies in adults. Canadian journal of diabetes, 37, pp.S72-S76.
*Gouveia, C.F. and Chowdhury, T.A., 2013. Managing hyperglycaemic emergencies: an illustrative case and review of recent British guidelines. Clinical Medicine, 13(2), pp.160-162.
Greetham, B., 2014. How to write your undergraduate dissertation. Palgrave Macmillan. London.
Gregg, E.W., Chen, H., Wagenknecht, L.E., Clark, J.M., Delahanty, L.M., Bantle, J., Pownall, H.J., Johnson, K.C., Safford, M.M., Kitabchi, A.E. and Pi-Sunyer, F.X., 2012. Association of an intensive lifestyle intervention with remission of type 2 diabetes. Jama, 308(23), pp.2489-2496.
Griffiths, J., Fortune, G., Barber, V. and Young, J.D., 2007. The prevalence of post-traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive care medicine, 33(9), pp.1506-1518.
Grove, S.K., Burns, N. and Gray, J.R., 2014. Understanding nursing research: Building an evidence-based practice. Elsevier Health Sciences: Atlanta, GA (US)
Guh, D.P., Zhang, W., Bansback, N., Amarsi, Z., Birmingham, C.L. and Anis, A.H., 2009. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC public health, 9(1), p.88.
Guisado, R. and Arieff, A.I., 1975. Neurologic manifestations of diabetic comas: correlation with biochemical alterations in the brain. Metabolism, 24(5), pp.665-679.
Haber, J. and LoBiondo-Wood, G. eds., 2014. Nursing research: methods and critical appraisal for evidence-based practice. Mosby. Maryland Heights, Missouri (US).
Hackel, J., 2014. A case of a patient with hyperosmolar hyperglycemic state: Implications for nurse practitioners in primary care. Journal of the American Association of Nurse Practitioners, 26(11), pp.595-602.
Hardern, R.D. and Quinn, N.D., 2003. Emergency management of diabetic ketoacidosis in adults. Emergency medicine journal, 20(3), pp.210-213.
Health and Social Care Information Centre. National Diabetes Audit 2010-2011. Report 2: Complications and mortality. [online] Available at: http://www.content.digital.nhs.uk/catalogue/PUB12738/nati-diab-audi-11-12-mort-comp-rep.pdf Accessed 28 September 2017.
Henderson, D.C., Cagliero, E., Copeland, P.M., Louie, P.M., Borba, C.P., Fan, X., Freudenreich, O. and Goff, D.C., 2007. Elevated hemoglobin A1c as a possible indicator of diabetes mellitus and diabetic ketoacidosis in schizophrenia patients receiving atypical antipsychotics. The Journal of clinical psychiatry, 68(4), pp.533-541.
Higgins, C., 2001. Diagnosing diabetes: blood glucose and the role of the laboratory. British Journal of Nursing, 10(4), pp.230-236.
Hill, J., 2009. Reducing the risk of complications associated with diabetes. Nursing Standard, 23(25), pp.49-55.
Howson JM, Rosinger S, Smyth DJ, Boehm BO, Todd JA., 2011. Genetic analysis of adult-onset autoimmune diabetes. Diabetes 60:2645–53.
Jackson, J.C., Hart, R.P., Gordon, S.M., Hopkins, R.O., Girard, T.D. and Ely, E., 2007. Post-traumatic stress disorder and post-traumatic stress symptoms following critical illness in medical intensive care unit patients: assessing the magnitude of the problem. Critical Care, 11(1), p.R27.
Johansen, M.Y., MacDonald, C.S., Hansen, K.B., Karstoft, K., Christensen, R., Pedersen, M., Hansen, L.S., Zacho, M., Wedell-Neergaard, A.S., Nielsen, S.T. and Iepsen, U.W., 2017. Effect of an Intensive Lifestyle Intervention on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial. Jama, 318(7), pp.637-646.
Kisiel, M. and Marsons, L., 2009. Recognizing and responding to hyperglycaemic emergencies. British journal of nursing, 18(18).
Kitabchi, A.E., Hirsch, L.B., and Emmett, M. (2014) Diabetic ketoacidosis and hyperosmolar hyperglycemic state in adults: Epidemiology and Pathogenesis. [online]. Available to subscribers only at: www.uptodate.com. Not Accessed.
Kitabchi, A.E., Umpierrez, G.E., Fisher, J.N., Murphy, M.B. and Stentz, F.B., 2008. Thirty years of personal experience in hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. The Journal of Clinical Endocrinology & Metabolism, 93(5), pp.1541-1552.
Kitabchi, A.E., Umpierrez, G.E., Miles, J.M. and Fisher, J.N., 2009. Hyperglycemic crises in adult patients with diabetes. Diabetes care, 32(7), pp.1335-1343.
Kitabchi, A.E., Umpierrez, G.E., Murphy, M.B. and Kreisberg, R.A., 2006. Hyperglycemic crises in adult patients with diabetes. Diabetes care, 29(12), pp.2739-2748.
Kitabchi, A.E., Umpierrez, G.E., Murphy, M.B., Barrett, E.J., Kreisberg, R.A., Malone, J.I. and Wall, B.M., 2001. Management of hyperglycemic crises in patients with diabetes. Diabetes care, 24(1), pp.131-153.
*Lenahan, C.M. and Holloway, B., 2015. Differentiating between DKA and HHS. Journal of Emergency Nursing, 41(3), pp.201-207.
Maclaren N, Atkinson M. 1992. Is insulin-dependent diabetes mellitus environmentally induced? N Engl J Med 199 2; 327: 348–49.
*Maletkovic, J. and Drexler, A., 2013. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Endocrinology and metabolism clinics of North America, 42(4), pp.677-695.
Mazze, R.S., Sinnock, P., Deeb, L. and Brimberry, J.L., 1985. An epidemiological model for diabetes mellitus in the United States: five major complications. Diabetes research and clinical practice, 1(3), pp.185-191.
*McCombs, D.G., Appel, S.J. and Ward, M.E., 2015. Expedited diagnosis and management of inpatient hyperosmolar hyperglycemic nonketotic syndrome. Journal of the American Association of Nurse Practitioners, 27(8), pp.426-432.
McNaughton, C.D., Self, W.H. and Slovis, C., 2011. Diabetes in the emergency department: acute care of diabetes patients. Clinical Diabetes, 29(2), pp.51-59.
Misra, S. and Oliver, N.S., 2015. Diabetic ketoacidosis in adults. BMJ, 351 pp1-8, [online]: Available at: http://www.bmj.com/content/351/bmj.h5660 Accessed 25 September, 2017.
*Miyares, M.A., Zlicha, A. and Rodriguez, J.N., 2013. Development and implementation of a hyperglycemic emergency protocol. American Journal of Health-System Pharmacy, 70(18), pp.1567-1577.
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group, 2009. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. [online] Available at: https://doi.org/10.1371/journal.pmed.1000097 Accessed 21 September 2017.
Morales, A.E. and Rosenbloom, A.L., 2004. Death caused by hyperglycemic hyperosmolar state at the onset of type 2 diabetes. The Journal of pediatrics, 144(2), pp.270-273.
Mulnier HE, Seaman HE, Raleigh VS, Soedamah-Muthu SS, Colhoun HM, Lawrenson RA. 2006. Mortality in people with Type 2 diabetes in the UK. Diabet Med 23: 516–521.
Muoio, D.M. and Newgard, C.B., 2008. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and [beta]-cell failure in type 2 diabetes. Nature reviews. Molecular cell biology, 9(3), p.193.
National Health Service (NHS) England 2014 The Five Year Forward View. [online] available at: www.england.nhs.uk/five-year-forward-view. Accessed 16 September . 2017.
National Health Service (NHS) Information Centre. 2011. NHS reference costs, 2010-2011.[online] Available at: http://data.gov.uk/dataset/nhs-reference-costs-2010-11 Accessed 19 September 2017
National Health Service (NHS) UK, 2017. Type 2 Diabetes. Food and keeping active [online] Available at https://beta.nhs.uk/conditions/type-2-diabetes/food-and-keeping-active/ Accessed 12 September 2017.
National Institute of Health and Care Excellence (NICE), 2011. Diabetes in Adults (QS6) [online] Available at: https://www.nice.org.uk/Search?ps=50&q=diet+and+type+2+diabetes Accessed 19 September 2017
National Institute of Health and Care Excellence (NICE), 2016a. Transition from children’s to adults’ services for young people using health or social care services (NG43) [online] Available at: https://www.nice.org.uk/guidance/ng43/documents/transition-from-childrens-to-adults-services-costing-consequences2. Accessed 13 September 2017
National Institute of Health and Care Excellence (NICE), 2016b Evidence Search [online] Available at: https://www.evidence.nhs.uk/Search?ps=50&q=casp+tools Accessed 21 September, 2017
National Institute of Health and Care Excellence (NICE), 2015a. Type 1 diabetes in adults: diagnosis and management (NG17). [online] Available at https://www.nice.org.uk/guidance/ng17 Accessed 26 September, 2017.
National Institute of Health and Care Excellence (NICE), 2015b. Type 2 diabetes in adults: management [online] Available at: https://www.nice.org.uk/guidance/ng28 Accessed 20 September 2017.
National Institute of Health (NIH), 2016. Fact Sheet: Medline [online] Available at: https://www.nlm.nih.gov/pubs/factsheets/medline.html Accessed 22 September, 2017
Nazarko, L., 2009. Causes and consequences of diabetes. British Journal of Healthcare Assistants, 3(11).
Nursing and Midwifery Council, 2015. The Code Professional standards of practice and behaviour for nurses and midwives [online] available at: https://www.nmc.org.uk/globalassets/sitedocuments/nmc-publications/nmc-code.pdf Accessed 12 September, 2017
Nwaneri, C., Cooper, H. and Bowen-Jones, D., 2013. Mortality in type 2 diabetes mellitus: magnitude of the evidence from a systematic review and meta-analysis. The British Journal of Diabetes & Vascular Disease, 13(4), pp.192-207.
Nyenwe, E.A. and Kitabchi, A.E., 2011. Evidence-based management of hyperglycemic emergencies in diabetes mellitus. Diabetes research and clinical practice, 94(3), pp.340-351.
Olokoba, A.B., Obateru, O.A. and Olokoba, L.B., 2012. Type 2 diabetes mellitus: a review of current trends. Oman medical journal, 27(4), p.269.
Oxman, A.D., Cook, D.J., Guyatt, G.H., Bass, E., Brill-Edwards, P., Browman, G.,Detsky, A., Farkouh, M., Gerstein, H., Haines, T. and Haynes, B., 1994. Users' guides to the medical literature: VI. How to use an overview. Jama, 272(17), pp.1367-1371.
Parahoo, K., 2014. Nursing research: principles, process and issues. Palgrave Macmillan. Basingstoke, UK.
Paterson, K.R., 1990. Hyperglycaemia and Hypoglycaemia. In Diabetes in Elderly People (pp. 69-81). Springer, US.
Polit-O'Hara, D. and Beck, C.T., 2006. Essentials of nursing research: Methods, appraisal, and utilization (Vol. 1). Lippincott Williams & Wilkins, Philadelphia, US.
Pørksen, N., Nyholm, B., Veldhuis, J.D., Butler, P.C. and Schmitz, O., 1997. In humans at least 75% of insulin secretion arises from punctuated insulin secretory bursts. American Journal of Physiology-Endocrinology And Metabolism, 273(5), pp.E908-E914.
Rapp, S.R., Luchsinger, J.A., Baker, L.D., Blackburn, G.L., Hazuda, H.P., Demos‐McDermott, K.E., Jeffery, R.W., Keller, J.N., McCaffery, J.M., Pajewski, N.M. and Evans, M., 2017. Effect of a Long‐Term Intensive Lifestyle Intervention on Cognitive Function: Action for Health in Diabetes Study. Journal of the American Geriatrics Society, 65(5), pp.966-972.
Reaven, G.M., Bernstein, R., Davis, B. and Olefsky, J.M., 1976. Nonketotic diabetes mellitus: insulin deficiency or insulin resistance? The American journal of medicine, 60(1), pp.80-88.
Rosenbloom, A.L., 2010. Hyperglycemic hyperosmolar state: an emerging pediatric problem. The Journal of pediatrics, 156(2), pp.180-184.
Salahuddin, M. and Anwar, M.N., 2011. Study on effectiveness of guidelines and high dependency unit management on diabetic ketoacidosis patients. Journal of Postgraduate Medical Institute (Peshawar-Pakistan), 23(2).
*Scott, A.R., 2015. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabetic Medicine, 32(6), pp.714-724.
Scott, A., Claydon, A. 2012. The management of hyperosmolar hyperglycaemic state (HHS) in adults with diabetes. Joint British Diabetes Societies Inpatient Care Group [online] Available at: www.diabetes.nhs.co.uk Accessed 10 September, 2017
Shea, B.J., Grimshaw, J.M., Wells, G.A., Boers, M., Andersson, N., Hamel, C., Porter, A.C., Tugwell, P., Moher, D. and Bouter, L.M., 2007. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC medical research methodology, 7(1), p.10.
Sillitoe, L. (2014) Request for information: cost of chest X rays and CT scans. [online] Available at https://www.whatdotheyknow.com/request/199758/response/498958/attach/3/FOI23023%20ResponseAttachment%20Deakin.pdf?cookie_passthrough=1 Accessed 16 September 2017.
Simmons, R.K., Echouffo-Tcheugui, J.B., Sharp, S.J., Sargeant, L.A., Williams, K.M., Prevost, A.T., Kinmonth, A.L., Wareham, N.J. and Griffin, S.J., 2012. Screening for type 2 diabetes and population mortality over 10 years (ADDITION-Cambridge): a cluster-randomised controlled trial. The Lancet, 380(9855), pp.1741-1748.
Sommese, L., Zullo, A., Mancini, F.P., Fabbricini, R., Soricelli, A. and Napoli, C., 2017. Clinical relevance of epigenetics in the onset and management of type 2 diabetes mellitus. Epigenetics, pp.1-15.
Spiteri, L., 1998. A simplified model for facet analysis: Ranganathan 101. Canadian journal of information and library science, 23(1-2), pp.1-30.
The Environmental Determinants of Diabetes in the Young (TEDDY) Study Group, 2008. The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann NY Acad Sci 2008; 1150: 1–13.
The Cochrane Library 2017. The Cochrane database of systematic reviews. [online] http://www.cochranelibrary.com/cochrane-database-of-systematic-reviews/ accessed 24th September, 2017
*Van Ness-Otunnu, R. and Hack, J.B., 2013. Hyperglycemic crisis. The Journal of emergency medicine, 45(5), pp.797-805.
Wachtel, T.J., Silliman, R.A. and Lamberton, P., 1987. Predisposing factors for the diabetic hyperosmolar state. Archives of internal medicine, 147(3), pp.499-501.
Walliman, N., 2004. Your undergraduate dissertation: the essential guide for success. Sage. Thousand Oaks, California.
Wang, J.Y., Wang, C.Y., Huang, Y.S., Chen, P.F., Huang, K.Y., Chou, P., Lian, W.C. and Lee, C.C., 2014. Increased risk of ischemic stroke after hyperosmolar hyperglycemic state: a population-based follow-up study. PloS one, 9(4), p.e94155.
Williams, G., Pickup, JC (2004). Handbook of Diabetes, 3rd Ed. Blackwell Publishing, Oxford.
Winter, W.E. and Pittman, D., 2013. The clinical application of islet autoantibody testing for the diagnosis of autoimmune diabetes. MLO. Med Lab Obs, 45, pp.16-20.
World Health Organization, 2013. Diabetes: Factsheet 312. [online] available at: https://web.archive.org/web/20130826174444/http://www.who.int/mediacentre/factsheets/fs312/en/ Accessed 18 September 2017
Appendices
Appendix 1: Preferred Reporting Instrument for Systematic reviews and Meta Analyses (PRISMA) diagram illustrating the article selection procedure.
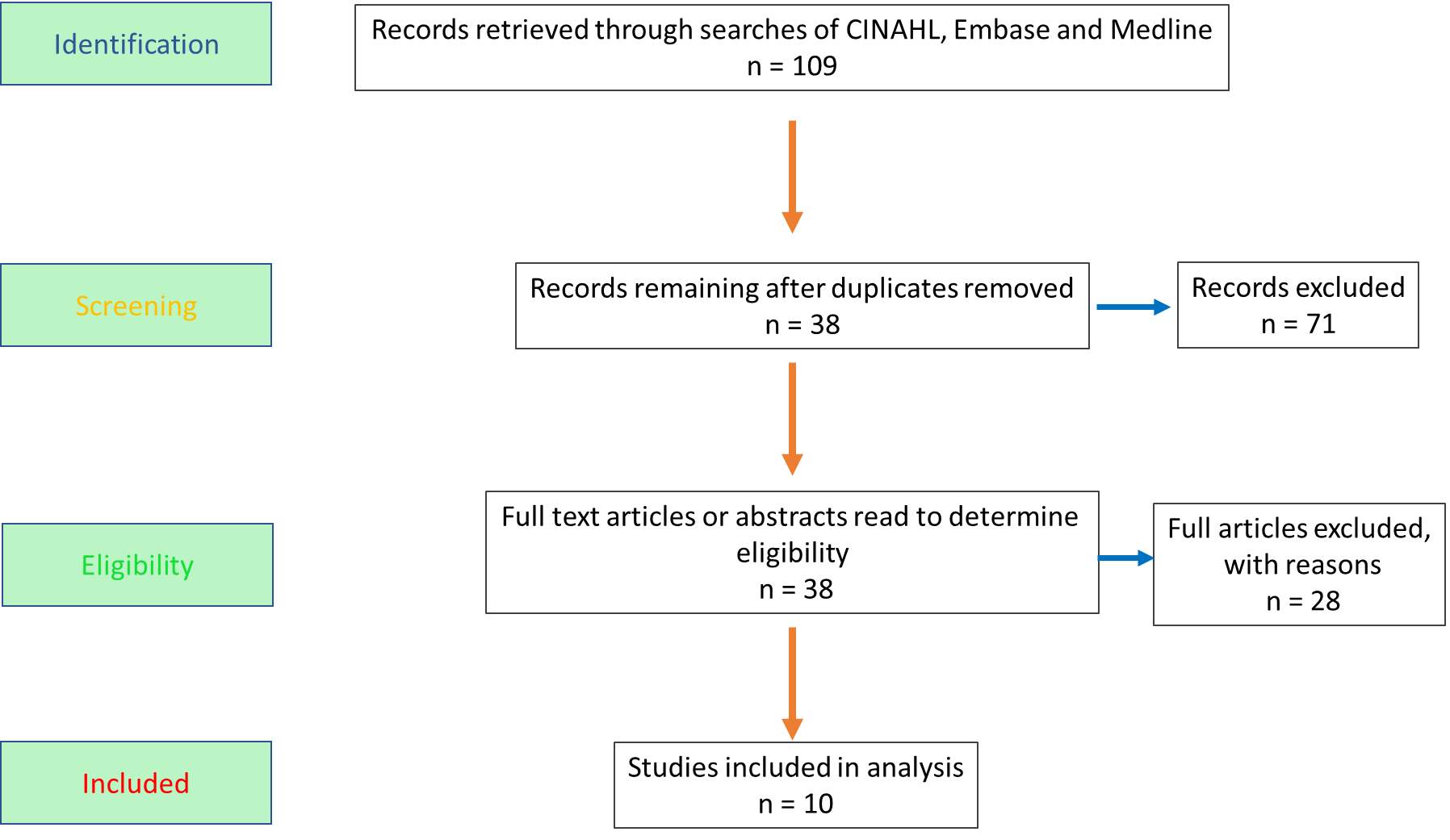
Appendix 2: Non-selected studies.
|
Table 2A: Studies retrieved from the three database searches but not included for review, for the reasons stated. |
|
|
Bibliographic reference |
Reason study not selected for review |
|
Abud, L.G., Abud, T.G., Queiroz, R.M., Pietroni, G.S. and Abud, D.G., 2016. Diabetic hemichorea-hemiballismus with nonketotic hyperglicemia: a rare cause of hyperkinetic movement disorders. Arquivos de neuro-psiquiatria, 74(4), pp.354-355. |
A case study outlining the presentation of HHS accompanied by hyperkinetic movement. |
|
Agrawal, Y., Lingala, K., Tokala, H. and Kalavakunta, J.K., 2016. Antipsychotic therapy-induced new onset diabetic ketoacidosis. American journal of therapeutics, 23(6), pp.e1944-e1945. |
Describes the iatrogenic development of HHS, as opposed to treatment. |
|
Berk, J., Wade, R., Baser, H.D. and Lado, J., 2015. Case report: severe reversible cardiomyopathy associated with systemic inflammatory response syndrome in the setting of diabetic hyperosmolar hyperglycemic non-ketotic syndrome. BMC cardiovascular disorders, 15(1), p.123. |
This is a single case report that discusses cardiomyopathy as a complication of HHS, and discusses counteracting systemic inflammatory response syndrome rather than HHS. |
|
Billich, C., Huber, S. and Schmitz, B., 2015. CT and MRI changes in the basal ganglia non-ketoacidosis hyperglycemic hemichorea-hemiballism syndrome. RöFo: Fortschritte auf dem Gebiete der Röntgenstrahlen und der Nuklearmedizin, 187(12), p.1128. |
Discusses the value of CT and MRI scanning in the diagnosis of DKA and HHS |
|
Chang, L.H., Guo, Y.W. and Chen, H.S., 2016. Hyperglycemic Hyperosmolar Status Is Associated with Subsequent All‐Cause Mortality in Elderly Adults with Type 2 Diabetes Mellitus. Journal of the American Geriatrics Society, 64(11). |
Discusses HHS as a complication of diabetes, does not discuss treatment. |
|
Ghorani, E., Evans, M.L. and Simmons, D., 2016. HHS in type 1 diabetes associated with medication overdose: can counter‐regulatory hormone suppression prevent diabetic ketoacidosis? Practical Diabetes, 33(2), pp.54-56. |
Discusses HHS treatment as a secondary condition caused by medication overdose. |
|
Hackel, J., 2014. A case of a patient with hyperosmolar hyperglycemic state: Implications for nurse practitioners in primary care. Journal of the American Association of Nurse Practitioners, 26(11), pp.595-602. |
This is a case study of a male patient with a history of renal failure but no previous diagnosis of diabetes who presented with HHS as the first symptom of subsequently diagnosed type 2 DM. |
|
Hirayama, I., Hiruma, T., Ueda, Y., Doi, K. and Nakajima, S., 2016. Hamman syndrome: pneumomediastinum combined with hyperosmolar hyperglycemic state. The American journal of emergency medicine, 34(10), pp.2058-e1. |
A case study discussing the presentation of pneumomediastinum as symptom of HHS. |
|
Jensen, K., Steinthorsdottir, K.J. and Brandt, B., 2013. In-hospital cardiac arrest due to unobserved steroid-induced hyperglycaemic hyperosmolar syndrome. Ugeskrift for laeger, 175(15), pp.1044-1045. |
This case study describes the iatrogenic cause of HHS arising from the treatment of a patient suffering from oesophageal cancer with corticosteroids. |
|
Koma, K.K.B.H.H., Hyperosmolar Nonketotic State Associated with Quetiapine. |
No full reference or abstract available, title indicates that the article describes the iatrogenic development of HHS, as opposed to treatment. |
|
Kruljac, I., Rinčić, G. and Pećina, H.I., 2016. Hyperosmolar coma in a patient with hypothalamic Langerhans cell histiocytosis. Endocrine, 52(1), pp.176-177. |
A case study of a patient suffering from hyperosmolar coma associated with a rare endocrine disorder that is often a complication of diabetes insipidus. |
|
Lee, J.H., Kim, Y.A., Moon, J.H., Min, S.H., Song, Y.S. and Choi, S.H., 2016. Expressive aphasia as the manifestation of hyperglycemic crisis in type 2 diabetes. The Korean journal of internal medicine, 31(6), p.1187. |
A case study describing the presentation of a middle aged man with a 5 day history of aphasia and subsequently found to be experiencing HHS. |
|
Lee, I.S., Lee, T.W., Chang, C.J., Chien, Y.M. and Lee, T.I., 2015. Pheochromocytoma presenting as hyperglycemic hyperosmolar syndrome and unusual fever. Internal and emergency medicine, 10(6), pp.753-755. |
A case study of a 68 year old woman with a pheochromcytoma (a rare tumour that can generate physiological inflammatory responses similar to those produced in sepsis who has undiagnosed DM, but who presented with HHS. |
|
Lee, S.P., See, T.T. and Kuo, K.H., 2013. Central pontine myelinolysis in a chronic alcoholic patient with hyperglycemic hyperosmotic state. Acta Neurol Taiwan, 22(3), pp.142-143. |
A case study of a 38 year old diabetic man whose type 2 diabetes had been poorly controlled, which, together with alcoholism, precipitated HHA with central pontine myelinolysis |
|
Lontchi‐Yimagou, E., Nguewa, J.L., Assah, F., Noubiap, J.J., Boudou, P., Djahmeni, E., Balti, E.V., Atogho‐Tiedeu, B., Gautier, J.F., Mbanya, J.C. and Sobngwi, E., 2017. Ketosis‐prone atypical diabetes in Cameroonian people with hyperglycaemic crisis: frequency, clinical and metabolic phenotypes. Diabetic Medicine, 34(3), pp.426-431. |
Discusses treatment of DKA as opposed to HHS. |
|
Lu, C.L., Chang, H.H., Chen, H.F., Ku, L.J.E., Chang, Y.H., Shen, H.N. and Li, C.Y., 2016. Inverse relationship between ambient temperature and admissions for diabetic ketoacidosis and hyperglycemic hyperosmolar state: A 14-year time-series analysis. Environment international, 94, pp.642-648. |
An epidemiological study that examines seasonal variation and temperatures as factors that contribute to the risk of developing DKA or HHS. |
|
Maggiore, W.A., 2013. Highs & lows: recognizing & treating hypoglycemia, hyperglycemia & other diabetes-related health problems. JEMS: a journal of emergency medical services, 38(12), p.44. |
A single case study, therefore findings and recommendations not generalizable. |
|
Maust, M.S., Muramatsu, R.S., Egan, K. and Ahmed, I., 2015. Perphenazine-Associated Hyperosmolar Hyperglycemic State. Journal of clinical psychopharmacology, 35(4), pp.485-486. |
Letter, no peer reviewed article, and describes an iatrogenic cause of HHS (antipsychotic medication). |
|
Milano, A., Tadevosyan, A., Hart, R., Luizza, A. and Eberhardt, M., 2016. An uncommon complication of hyperosmolar hyperglycemic state: bilateral above knee amputations. The American journal of emergency medicine, 34(2), pp.341-e1. |
Discusses bilateral knee amputation as a complication of HHS, does not discuss treatment. |
|
Olivero Sr, J.J., 2016. Electrolyte Games for Rainy Afternoons. Methodist DeBakey cardiovascular journal, 12(3), pp.184-185. |
Not a peer reviewed article. This is an exercise targeted at medical students and clinicians designed to test knowledge of electrolyte imbalance in specific conditions. |
|
Pasquel, F.J. and Umpierrez, G.E., 2014. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care, 37(11), pp.3124-3131. |
No information is presented relating to treatment of HHS referenced after 2011 and no recommendations are made. |
|
Ray, S., Howlader, S., Chakraborty, S., Chakraborty, P.P. and Ghosh, S., 2015. Hemichorea-hemiballism as the first presentation of type 2 diabetes. Clinical Diabetes, 33(2), pp.87-89 |
Discusses a condition which is a complication of HHS. |
|
Siwakoti, K., Giri, S. and Kadaria, D., 2017. Cerebral edema among adults with diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome: Incidence, characteristics, and outcomes. Journal of diabetes, 9(2), pp.208-209. |
Research letter. The aetiology and incidence of stroke in patients as a result of hyperglycaemic crisis is summarised. |
|
Shrivastava, V., Burji, N.P., Basumatary, L.J., Das, M., Goswami, M. and Kayal, A.K., 2013. Etiological profile of epilepsia partialis continua among adults in a tertiary care hospital. Neurology India, 61(2), p.156. |
Discusses HHS as a complication of epilepsy, does not discuss treatment. |
|
Sinson, N., Veen, J.J., Brown, P. and Creagh, F., 2016. Acute pyelonephritis with renal vein and inferior vena cava thrombosis and pulmonary emboli in poorly controlled type 2 diabetes and HHS. Practical Diabetes, 33(5), pp.167-169. |
Discusses the treatment of a condition arising as a complication of HHS rather than specific HHS management. |
|
Spyridoulias, A. and Riaz, M.S., 2016. Myxoedema coma in the setting of hyperglycaemic hyperosmolar state. BMJ case reports, 2016, p.bcr2015213411. |
A case study in which a patient with a rare thyroid disorder experienced coma induced by decompensated hyperthyroidism rather than diabetes. |
|
Wang, Y.C., Gao, L.C., Xu, H., Qu, X.Z., Wang, Y., Lou, X.Q. and Guo, H., 2015. A successfully treated case of hypopituitarism complicated with hyperosmolar hyperglycaemic state and rhabdomyolysis. Scottish medical journal, 60(2), pp.e7-e10. |
A case study in which HHS and rhabdomyolysis occurred as a complication of rare hypopituitarism. |
|
Wang, J.Y., Wang, C.Y., Huang, Y.S., Chen, P.F., Huang, K.Y., Chou, P., Lian, W.C. and Lee, C.C., 2014. Increased risk of ischemic stroke after hyperosmolar hyperglycemic state: a population-based follow-up study. PloS one, 9(4), p.e94155. |
An investigation into the risk of stroke following HHS and which found that HHS does increase the risk of subsequent ischemic stroke. |
Appendix 3: Data extraction (selected studies).
Recommendations are categorised into specific interventions in HHS management, as provided by the JBDS HHS care pathway. Where recommendations from the articles considered concur with 2012 JBDS guidelines, this is indicated by ‘As JBDS’. In instances in which there are differences, these are expounded upon.
|
Table 3A: Data extraction from selected studies that address the research question. A comparison with 2012 JBDS recommendations is in italics. |
|
1. Maletkovic and Drexler, (2013) (Review). |
|
Initial assessment: Biochemical screening to include serum phosphate and magnesium, arterial blood gas analysis, hepatic enzymes, serum ketones, cardiac enzymes, Haemoglobin A1C (HbA1C), coagulation profile and hepatic enzyme analysis. In addition, complete blood count, blood and urine cultures are advocated. In selected cases, the authors recommend a lumbar puncture and/or chest/brain/abdominal imaging depending upon clinical presentation, and Electrocardiogram and chest X rays should be conducted. These concur with the JBDS HHS care pathway, but include significantly more initial biochemical and physiological analysis. There are no investigations recommended by the JBDS that are not also recommended by these authors. Fluid replacement: Normal saline (N/S), which is 0.9% sodium chloride at 0-15 ml /kg over first hours. When patients euvolemic, switch to half N/S (0.45 sodium chloride) at 4-14 ml/kg. Supplement half N/S with 5% dextrose when blood glucose reduces below 300 mg/dL (17mmol/L). The JBDS does not advocate using a flat rate for fluid infusion, but instead to titrate the rate of infusions to serum osmolality in order to prevent too rapid fluid resuscitation which can lead to cerebral oedema and /or heart failure. The JBDS does advocate the change to half N/S as indicated above but specifies that this is appropriate if osmolality is increasing but the patient is euvolemic. Additionally, the JBDS HSS care pathway is divided into periods from 0-1, 1-6, 6-12, 12-24 and 24 hours onwards, in which specific directions are given depending upon serum osmolality, blood glucose and the patients level of consciousness. Insulin supplementation and blood glucose control: Low dose insulinshould be commenced one hour after the initiation of fluid replacement therapy. However, this should be delayed of the patient is hypokalaemic and the infusion rate should be based upon the patients calculated anion gap not serum glucose. Once the plasma glucose level is below 18mmmol/L the rate of insulin infusion should be decreased to avoid hypoglycaemia, and blood glucose maintained at 8-11 mmol/L until hypoglycaemia is resolved. The JBDS HHS care pathway recommendations specify in more detail that 0.05 units of insulin/kh/h should be commenced only of there is significant ketonaemia or ketonurea (which indicates a mixed picture of HHS and DKA) in the first hour. Insulin is recommended after fluid balance restored to maintain blood glucose between 10-15 mmol/L, a wider range than recommended by Maletkovic and Drexler, (2013) Bicarbonate supplementation: Not recommended because not supported by sufficient evidence. Bicarbonate is not addressed by JBDS HHS care pathway guidelines. Serum potassium: Correct for loss of potassium (caused by intracellular fluid shift in absence of insulin) once serum potassium is <5.3 mmol/L and maintain at 4-5 mmol/L. JBDS HHS care pathway guidelines aim to maintain serum potassium at between 3.5 mmol/L (less than 3.5 is hypokalaemic) and 6 mmol/L (hyperkalaemic). If serium potassium is over 5.5 mmol/L, no supplementation is advocated. Between 3.5 mmol/L and 5.5 mmol/L, potassium supplementation to IV fluids is recommended. If potassium is below 3.5 mmol/L , the patient requires a senior specialist review. Therefor,e these guidelines are more specific than those offered by Maletkovic and Drexler and, crucially, identify the need for specialist input if blood potassium levels are < 3.5 mmol/L |
|
2. Van Ness-Ottunu and Hack, (2012) (Review) |
|
Initial assessment: As Maletkovic and Drexler, (2013) with the addition of toxicology screening (some drugs such as aspirin and antipsychotics can induce HHS (Henderson et al., 2007, Abate & Buttaro, 2015) and pregnancy testing. See above (1) for comparison of initial assessment with recommendations made by the JBDS HHS care pathway. Fluid replacement: IV N/S at 15-20 ml/kg/h should be commenced immediately and hydration status measured hourly. After fluid resuscitation, corrected serum sodium should guide The selection of fluid for resuscitation. For hyponatraemia, continue N/S at 250-500 ml/h. If corrected sodium indicates hypernatraemia, reduce to half N/S at the same rate. Aim for urine output of 0.5-1 ml/kg/h. Tailored management is recommended for elderly patients with cardiac or renal disease. These guidelines differ to those recommended by the JBDS outlined above (1), and in addition use calculated serum sodium to direct fluid replacement whereas the JBDS use osmolality. No minimum urine output is specified by the JBDS but hourly measurement of fluid balance which included urine output is recommended. Insulin supplementation and blood glucose control: After initial IV N/S bolus,IV insulin should be commenced at 0.14 units/kg/h. Administer bolus doses of insulin if blood glucose does not decrease by 10% after the first hour. An expected decrease in blood glucose should be 2.7-3.2 mmol/L/h. These recommendations differ from those of the JBDS as outlined above (1). Although the JBDS HHS pathway does not specify an ideal reduction rate for blood glucose, it does stipulate that this should not exceed 5 mmol/L/h. Bicarbonate supplementation: As above (1) Serum potassium: Monitor every 2 hours, delay insulin infusion until serum potassium >3.3 mmol/L. When serum potassium is 3.3-5.5 mmol/L, supplement IV fluids with potassium to maintain serum potassium between 4 and 5 mmol/L In common with article (1) above, the differences between these guidelines and those of the JBDS HHS care pathway apply. However, these authors concur with the JBDS in recommending roughly the same limits for the maintenance of plasma potassium concentration and appropriate action to be take regarding potassium supplementation. In common with article 1, these authors do not flag up the need for senior review if potassium levels fall below 3.5 mmol/L and in addition regard 3.3 mmol/L as the acceptable lower limit. |
|
3. Gouveia and Chowdhury, 2013 (review, which references 2012 JBDS guidelines) |
|
Initial assessment: Identification of causes, extent of fluid imbalance, serum osmolality consider anticoagulation, daily foot assessment instigated. These authors have summarised JBDS HHS care pathway guidelines and, in doing so, have not provided specific details of biochemical and physical investigations that comprise initial assessment. For example, in stating that the underlying cause should be identified, they have not listed the tests that could be performed to ascertain this, such as blood and urine cultures. However, the JBDS do not provide as much detail as some authors (for example, Maletkovic and Drexler, (2013)) Fluid replacement: Aim for a positive fluid balance of 3-6 L within the first 12 hours and replace all lost fluid by 24 hours. Use half N/S if osmolality is not falling but patient is euvolemic. Assess frequently for signs of fluid overload. This concurs with all JBDS HHS pathway guidelines but presented in a much summarised form, and thus lack the detail provided by the JBDS. Insulin supplementation and blood glucose control: reproduced as per JBDS HHS care pathway guidelines. Bicarbonate Supplementation: As above (1) Serum potassium: Not addressed. |
|
3. Corwell et al., 2014 (review). |
|
Initial Assessment: No recommendations made and ‘assessment’ as such is not delineated. However, the text refers to the need for information relating to the degree of dehydration (using serum osmolality), obtaining blood glucose and sodium levels and conducting arterial blood gas analysis. This lack of a formal assessment is not reflected by JBDS HHS pathway guidelines and the information presented lacks the specificity offered by the JBDS. Fluid replacement: 1L of N/S to be infused over 30-60 minutes to provide 15-20 mL/kg over the first 2 hours, then another 2L over the following 2-6 hours and another 2L over 6-12 hours. This replaces 50% of lost fluid over the first 12 hours. Consider transferring to half N/S if hyponatraemia develops. Maintaining circulating volume takes precedence over correcting serum osmolality. This contradicts the recommendations of the JBDS HHS care pathway which titrates fluid resuscitation to serum osmolality and, crucially, does not regard this as less important than regaining serum volume. Insulin supplementation and blood glucose control: Insulin therapy should not be commenced immediately, but when it is commenced, 0.14 units/kg/h is advocated if a bolus dose has not been given. A drop in circulating blood glucose of 2.7-3.2 mmol/L/h is desirable (concurring with Van Ness-Ottunu and Hack, (2012) and JBDS guidelines. The latter state ‘no more than 5.0 mmol/L/h’. When plasma glucose falls to 14-17 mmol/L, Corwell et al., 2014 reports that infusion of dextrose containing fluid should be commenced and suggests 5% dextrose in half N/S at 150-250 ml/h. JBDS HHS care pathway guidelines contradict this advice and advocate commencing insulin initially at 0.05 units/kg/h only if there is evidence of ketones, indicative of some element of DKA. Rather than recommending a flat rate of insulin infusion, the JBDS recommend aiming to maintain blood glucose within the range of 5-10 mmol/L, and suggest commencing 5% or 10% dextrose if blood glucose falls below 14 mmol/L AND continuing with N/S at 125ml /h Bicarbonate supplementation: As above (1) Serum potassium: If serum potassium is < 3.3-3.5 mmol/L, insulin supplementation should be stalled. If serum potassium < 3.3 mmol/L, the addition of potassium to half N/S is advocated. If serum potassium is within normal range, 20-30 mmol of potassium can be added to each L of fluid. This reflects JBDS recommendations as outlined by paper (2) above. |
|
4. Dhatariya (2014), (review, which references 2012 JBDS guidelines, of which Ketan Dhatariya was a contributing author). |
|
All recommendations for initial therapy and treatment are as per 2012 JBDS HHS care pathway guidelines (see appendix 4). |
|
5. Lenahan and Holloway, 2015 (review) |
|
Initial Assessment: Patient assessment as such is not specified, nor is biochemical testing. Rather, the authors suggest that analysis should be conducted that is specific to the patients’ presentation and past medical history. They should include screening for pregnancy, infection and toxicology screen. Arterial blood testing, testing for blood and urine ketones are inferred, as are tests for serum osmolality and plasma glucose levels. Comparison to JBDS HHS care pathway guidelines is as for article (3) above. Fluid replacement: Give a fluid bolus of 500ml and continuous infusion of 1-2 L over the first 2h.Replace half of estimated fluid loss over the first 12 hours. Serum Osmolality should be reduced to <315 mOsm/kg In comparison to JBDS HHS care pathway guidelines, this lacks detail and the initial fluid resuscitation guidelines do not concur, nor is the type of fluid to be infused specified. Serum Osmolality baseline is not explicitly stated by the JBDS but 320 mOsmol/kg or above is stated as diagnostic for HHS, implying that below this would be a suitable target, concurring with Lenahan and Holloway (2015) . Other comments relating to fluid resuscitation that apply are as for article (1) above. Insulin supplementation and blood glucose control: Loadingdose of 0.1 units/kg/h, if blood glucose does not decrease by 10% in the first hour, give a second loading dose of 0.14 units/kg/h, OR provide fluid replacement and if blood glucose does not reduce by 2.7-3.9 mmol/L/h give a bolus of 0.1 units/kg of insulin. JBDS HHS guidelines offer more specific detail in relation to regimes of insulin administration and glucose supplementation, and provide a target range for glucose concentration, which is not specified by Lenahan and Holloway (2015). The latter authors provide no indication of the level of blood glucose that equates to hypoglycaemia that should, according to the JBDS, trigger referral to a diabetic specialist, in addition, insulin administration recommended by the JBDS contradicts giving a loading dose of insulin suggested by Lenahan and Holloway (2015) and the JBDS do not advocate subsequent bolus doses of insulin. Crucially, giving insulin before adequate fluid resuscitation causes a shift of water and glucose from the extracellular to the intracellular fluid, causing vascular collapse (Scott and Claydon, 2012) and is thus not advocated by the JBDS. Bicarbonate administration: As above, study (1). Potassium: Treat after patient haspassed urine, if potassium is <5.2 mmol/L supplement fluids with potassium at infusion rate of20-30 mmol/L/h This does not reflect JBDS HHS care pathway recommendations. These are, for serum potassium within the range 3.5-5.5 mmol/L, supplement fluids with Potassium at 40 mmol/L. |
|
6. Scott (2015), (review, which references 2012 JBDS guidelines, of which Adrian Scott was one of the two the main authors) |
|
Initial Assessment: Identify the extent of water and sodium deficit, assess cognitive impairment, examine cardiac function (ECG), physical examination for indicators of the extent of dehydration (high urea due to dehydration may be visible on examination by ‘sunken eyes, furrows on the tongue and extreme weakness’. Determine serum osmolality and blood glucose. Determine is infection or sepsis is present through physical examination and C reactive protein assay, conduct a screen of coagulation factors to determine the risk of thromboembolism but provide all patients with prophylactic anticogulation therapy. Measure serum lactate and ketones and electrolytes including potassium, assess need for high dependency admission. This advice expounds upon the JBDS HHS care pathway guidelines and provides a rationale for the various tests suggested. It would provide a useful supplement to the HHS care pathway, and provides assessment comparable in terms of detail to that suggested by Maletkovic and Drexler, (2013) and Van Ness-Ottunu and Hack, (2012). Fluid replacement: All recommendations are as per 2012 JBDS HHS care pathway guidelines (given in appendix 3 of the article), but with more detailed rationale for interventions and some additional details. These include the aim to not exceed a fall in serum sodium of more than 10 mmol/L over 34 h, and advice that complete normalization of fluid and electrolyte balance may take up to 72h. Insulin supplementation and blood glucose control: Start insulin at time zero only if ketonemia is present. If Ketones are <1mmol/L, do not start insulin. A rationale is provided for this (starting insulin too soon may result in cardiovascular collapse). All other recommendations are as per JBDS HHS care pathway guidelines, but with evidence based rationales provided. Bicarbonate administration: Not addressed, (nor is bicarbonate addressed by JBDS HHS care pathway guidelines.) Potassium: Guidelines are reproduced from the JBDS HHS care pathway with no further comments or recommendations offered. |
|
7. McCombs et al., 2015 (review) |
|
Initial Assessment: The authors do not delineate ‘assessment’ as such but the following is suggested by the text relating to ‘management’: Determine cause/precipitating factors (HbA1C assay to indicate extent of diabetes control, C reactive peptides to indicate infection), measure serum glucose, osmolality, bicarbonate, anion gap, ketones and assess extent of dehydration (volume depletion). As with Corwell, 2014 (article 3) this information lacks specific details and the comparison to comments JBDS HHS care pathway guidelines are as discussed above in relation to Corwell, 2014. Fluid replacement: No recommendations are offered other than that N/S should be the principle fluid administered to achieve rehydration. Changing to half N/S is mentioned when vital signs and electrolytes have stabilized but this is referenced to a non-peer reviewed, online, subscriber only source. Compared to JBDS HHS care pathway guidelines, the information presented does concur but lacks the detail provided by the JBDS and can be regarded as an overview, only. Lack of attention to the importance of serum osmolality as a guide to rehydration is a significant deficiency in this article. Insulin supplementation and blood glucose control: Commencement of insulin is not discussed, but the authors assert that when blood glucose reduces to 14 mmol/L, the rate of insulin should be decreased to 2-3 units/h and dextrose (at an unspecified concentration) added to IV fluids. Further suggestions are that 50g of dextrose is give every 8 hours and insulin adjusted appropriately. This is not referenced. These suggestions do not concur with the JBDS HHS care pathway guidelines. And specific (unreferenced) interventions are contradictory. For example, The action taken when blood glucose falls to 14mmol or less, which McCombs et al., (2015) should be managed by ‘reducing the rate of insulin infusion’, the JBDS recommend should be treated by adding 5% or 10% dextrose to N/S and infusing at 125 ml/h. Bicarbonate: as (1) above. Potassium: Not addressed. |
|
8. Miyares et al., 2013. (Protocol for the emergency management of HHS and DKA). |
|
Initial Assessment: Not addressed. The authors have developed a protocol that focuses upon the acute treatment of HHS only. The JBDS HHS care pathway includes a list of assessment criteria that are essential in determining the subsequent safe treatment of the patient. Fluid replacement: Fluid replacement should be provided at 250 ml/h using N/S with a change to half N/S at the clinicians’ discretion, supported by Kitabchi et al., (2009). Comparison with these guidelines and those of the JBDS HHS care pathway as stated for article 1 (Maletkovic and Drexler, 2013) Insulin supplementation and blood glucose control: No distinction is made for the treatment of HHS as opposed to DKA, rather it is left to the physicians’ discretion. Commencing immediate insulin infusion at 0.14 units/kg/h is recommended. Insulin infusion rates are titrated to blood glucose with advice to call upon senior medical advice if glucose levels reach or 18 mmol/L despite insulin treatment. If glucose levels fall below 4 mmol/L, practitioners are advised to follow the hypoglycaemic protocol and not to administer insulin. The addition of 5% dextrose to fluid replacement solution is recommended once glucose levels have fallen to 17 mmol/L with continuation of insulin titrated to avoid hypoglycaemia. In comparison to JBDS HHS pathway guidelines, commencement of IV insulin regardless of the presence of ketones in the blood or urine is contradictory and potentially dangerous (see comment relating to article (5) above). Interventions suggested should hypoclycaemia develop does concur with JBDS HHS pathway guidelines but have a slightly higher defining level of blood glucose (4mmol/L) Bicarbonate: as (1) above. Potassium: For serum potassium < 0.3 mmol/L administer 40 mmol of potassium, for 3.3-5.2 mmol/L, administer 20 mmol of potassium, and if >5.2 mmol/L, do not administer additional potassium. This advice contradicts the JBDS HHS care pathway in the action taken when serum potassium is 3.3-5.2 mmol/L, in which case Miyares et al., 2013 advocate administering 20 mmol of potassium. The JBDS recommend 40 mmol of potassium when the levels are between 3.5 and 5.5. In addition, the JBDS guidelines recommend senior review of the patient is hypokalaemic (potassium < 3.5 mmol/L). Miyares et al., in contrast, suggest giving 40 mmol of potassium. The JBDS guidelines recommend potassium supplementation up to serum potassium levels of 5.5 mmol/L whereas Miyares et al recommend ceasing potassium supplementation when serum levesl reach 5.2 mmol/L |
|
9. Goguen and Gilbert, 2013. The Canadian Diabetes Association (CDA) protocol comprising the clinical practice guidelines for the emergency treatment of HHS in adults. |
|
Initial Assessment: Extremely detailed, and includes determination of fluid loss, potassium and other electrolyte levels, extent of hyperosmolality, ECG, thyrotoxicosis screen, toxicology screen, previous medical history, coagulation screen, blood and urine cultures if infection suspected, HbA1C assay to ascertain previous glucose control, neurological status, anion gap, serum lactate if patient hypoxic, creatinine (to ascertain renal function), arterial or venous blood gas analysis depending upon severity of presentation, measurement of blood and urine ketones (may be very small in HHS). One confusing aspect of the entire protocol is that it combines DKA and HHS management, and the text describing HHS treatment refers to a figure (figure 1, p. S73) providing a management schematic diagram that is entitled ‘The management of Diabetic Ketoacidosis in adults’ These assessment criteria largely overlap with the JBDS guidelines, specified in the main body of the JBDS ‘Management of the HHS in adults’ text as opposed to the HHS care pathway in the later part of the document. However, the assessment suggested by the CDA is slightly more detailed, and includes testing for HbA1C, and a thyrotoxicosis screen, not suggested by the 2012 JBDS guidelines. This may be because Goguen and Gilbert, 2013 pay little attention to distinguishing between HHS and DKA Fluid replacement: N/S at 1-2 L/h, then N/S at 500ml/h for 4 hours, then 250 ml/h until euvolemic. Then, if corrected plasma sodium is normal or high and the fall in effective plasma osmolality is < 3mOsmol/kg/h, change to half N/S to replace fluid loss. If corrected plasma sodium is low or fall of effective plasma osmolality is at least 3 mOsmoll/kg/h, continue with N/S to a euvolemic state is achieved. These fluid resuscitation guidelines concur with those advocated by the JBDS HHS care pathway although the timescale over which infusion rates are applied varies by 2 hours (the JBDS HHS care pathway is divided into 6 hour constant infusion rates, as opposed to 4 recommended by the CDA, after the first hour and up to 12 hours. Both sets of guidelines emphasise the importance of using serum osmolality as a guide to infusion rate, however, and do not advocate inflexible application of the state infusion rates. In addition, the CDA guidelines and JBDS HHS care pathway confer flexibility in rate of infusion of resuscitation fluid in the first hour, depending upon degree of dehydration. The criteria for change to half N/S advocated the CDA accords with those applied by the JBDS. Insulin supplementation and blood glucose control: The supplementation of insulin in HHA and control of blood glucose is mentioned in the text but the recommendations are unclear and lack detail. For example, although the authors state (in common with the JBDS) that once plasma glucose has fallen to 14mmol/L, IV glucose should be provided, no indication is provided regarding the fluid carrier (5% or 10% dextrose). The recommendations by the CDA for insulin supplemnation and blood glucose lack sufficient detail to be applied to practice, unlike JBDS HHS care pathway guidelines that are more prescriptive when recommending insulin supplementation and glucose control. Bicarbonate: Not addressed. Potassium: Maintain serum potassium at3.3- 5.5 mmol/L. Give 10-40 mmol of potassium immediately if the patient is hypokalaemic on presentation, withhold insulin if plasma potassium is 3.3 mmol/L or less. Commence potassium supplementation into IV fluids when plasma levels are less than 5.0 -5.5 mmol/L. These recommendations concur with those of the JBDS HHS care pathway although the limits for potassium supplementation are for 40mmol/L if serum potassium levels are 3.5-5.5 mmol/L. Unlike the CDA, the JBDS highlights the need for senior medical review of potassium levels fall below 3.5 mmol/L
|
Appendix 4: The JBDS HHS care pathway (reproduced directly from Scott and Claydon, 2012)
HHS care pathway
The Hyperglycaemic Hyperosmolar State (HHS) is a medical emergency. In the UK it is less common than diabetic ketoacidosis (DKA), though in areas with a high proportion of patients of African origin this may not be the case. HHS is associated with a significant morbidity and higher mortality than DKA and must be diagnosed promptly and managed intensively. The diabetes specialist team should be involved as soon as possible after admission.
For young people under the age of 16 years contact your paediatric diabetes service and refer to published paediatric guidelines for the management of HHS such as those by Zeitler (2011).
Diagnosis
The characteristic features of a person with HHS are:
• Hypovolaemia
• Marked hyperglycaemia (30 mmol/L or more) without significant hyperketonaemia (less than 3 mmol/L), ketonuria (2+ or less) or acidosis (pH greater than 7.3, bicarbonate greater than 15 mmol/L)
• Osmolality usually 320 mosmol/kg or more N.B. A mixed picture of HHS and DKA may occur
Assessment of severity
Patients with HHS are complex and often have multiple co-morbidities so require intensive monitoring.
Consider the need for admission to a high-dependency unit / level 2 environment, when one or more of the following are present:
• osmolality greater than 350 mosmol/kg
• sodium above 160 mmol/L
• venous ⁄ arterial pH below 7.1
• hypokalaemia (less than 3.5 mmol/L) or hyperkalaemia (greater than 6 mmol/L) on admission
• Glasgow Coma Scale (GCS) less than 12 or abnormal
• AVPU (Alert, Voice, Pain, Unresponsive) scale
• oxygen saturation below 92% on air (assuming normal baseline respiratory function)
• systolic blood pressure below 90 mmHg
• pulse over 100 or below 60 bpm
• urine output less than 0.5 ml/kg/hr
• serum creatinine >200 μmol/L
• hypothermia
• macrovascular event such as myocardial infarction or stroke
• other serious co-morbidity.
Goals of treatment
The goals of treatment of HHS are to treat the underlying cause and to gradually and safely:
• normalise the osmolality
• replace fluid and electrolyte losses
• normalise blood glucose.
Other goals include prevention of:
• arterial or venous thrombosis
• other potential complications e.g. cerebral oedema/ central pontine myelinolysis
• foot ulceration.
New principles
• Measure or calculate osmolality (2Na+ + glucose + urea) frequently to monitor treatment response.
• Use IV 0.9% sodium chloride solution as the principle fluid to restore circulating volume and reverse dehydration. Only switch to 0.45% sodium chloride solution if the osmolality is not declining despite adequate positive fluid balance.
• An initial rise in sodium is expected and is not in itself an indication for hypotonic fluids. Thereafter, the rate of fall of plasma sodium should not exceed 10 mmol/L in 24 hours.
• The fall in blood glucose should be no more than 5 mmol/L/hr. Low dose IV insulin (0.05 units/kg/hr) should be commenced once the blood glucose is no longer falling with IV fluids alone OR immediately if there is significant ketonaemia (3-hydroxy butyrate greater than 1 mmol/L).
• Assess foot risk score on admission.
A. Hour 1: Immediate management upon diagnosis: 0 to 60 minutes
T=0 at time intravenous fluids are commenced. If there is a problem with intravenous access critical care
support should be requested immediately.
• Commence IV 0.9% sodium chloride – 1 litre to run over 1 hour
o Consider more rapid replacement if SBP below 90 mmHg
o Caution in the elderly where too rapid rehydration may precipitate heart failure but insufficient may fail to reverse acute kidney injury
• Only commence insulin infusion (0.05 units/kg/hr) IF there is significant ketonaemia (3-hydroxy but yrate greater than 1 mmol/L) or ketonuria 2+ or more (i.e. mixed DKA and HHS)
• Clinical assessment of the patient:
o Does the history suggest sepsis/vascular event or a recent change in medication?
o Assess the degree of dehydration
o Examine for a source of sepsis or evidence of vascular event
o Mental state assessment
• Assess foot risk score – assume high risk if patient obtunded or uncooperative
o Ensure heels are off-loaded
o Ensure daily foot checks
• Investigations
o Capillary BG
o Venous plasma BG
o Urea and electrolytes
o Measured or calculated osmolality (2Na+ + glucose + urea)
o Venous blood gas
o Blood ketones and lactate
o Full blood count
o Blood cultures
o ECG
o CXR
o Urinalysis and culture
o CRP (if indicated)
• Establish monitoring regime appropriate to patient – generally hourly blood glucose (BG), Na+, K+, urea and calculated osmolality (2Na+ + glucose + urea) for the first 6 hours then 2 hourly if response satisfactory (a fall of 3-8 mosmol/kg/hr).
o Chart osmolality / glucose / sodium
o Continuous pulse oximetry
o Consider continuous cardiac monitoring
• Insert urinary catheter to monitor hourly urine output and calculate fluid balance.
• Ensure early senior review and/or inform specialist diabetes team
• Commence prophylactic LMWH
• Consider IV antibiotics if sepsis identified or suspected
B. 60 minutes to 6 hours
Aims
• To achieve a gradual decline in osmolality (3-8 mosmol/kg/hr)
o Using 0.9% normal saline aim to give a further 0.5 – 1 L/hr depending on clinical assessment of
dehydration / risk of precipitating heart failure and fluid balance (target is to achieve positive
fluid balance of 2-3 L by 6 hours)
o Measure glucose, urea and electrolytes hourly and calculate osmolality (2Na+ + glucose + urea)
Δ If plasma Na+ increasing but osmolality declining at appropriate rate, continue 0.9% sodium chloride
Δ If plasma Na+ increasing AND osmolality increasing (or declining at less than 3 mosmol/kg/hr) check fluid balance. If positive balance inadequate increase rate of infusion of 0.9% sodium chloride
Δ If osmolality increasing and fluid balance adequate, consider switching to 0.45% sodium chloride at same rate
Δ If osmolality falling at rate exceeding 8 mosmol/kg/hr consider reducing infusion rate of IV fluids and/or insulin (if already commenced).
o If blood glucose falling less than 5 mmol/L per hour check fluid balance.
Δ If positive balance inadequate, increase rate of infusion of 0.9% sodium chloride
Δ If positive fluid balance adequate, commence low dose IV insulin (0.05 units/kg/hr) or if already running, increase rate to 0.1 units/kg/hr
• To maintain potassium in the normal range
o Hypokalaemia (less than 3.5 mmol/L) and hyperkalaemia (greater than 6 mmol/L) are life threatening conditions and warrant senior review. They are less common in HHS than DKA but monitoring and replacement are essential
|
Potassium level in first 24 hr (mmol/L) |
Potassium replacement in infusion solution |
|
Over 5.5 |
Nil |
|
3.5 - 5.5 |
40 mmol/L |
|
Below 3.5 |
Senior review as additional potassium required |
• Avoidance of hypoglycaemia
o Aim to keep blood glucose 10-15 mmol/L in first 24 hours
o If blood glucose falls below 14 mmol/L commence 5% or 10% glucose at 125 ml/hr AND
CONTINUE 0.9% sodium chloride solution
• Monitor vital signs and chart Early Warning Score (EWS)
• Maintain accurate fluid balance chart (minimum urine output 0.5 ml/kg/hr)
C. 6 to 12 hours
The aim within this time period is to:
• Ensure that clinical and biochemical parameters are improving
o Continue charting blood glucose hourly; sodium and calculated osmolality 2 hourly
o Take appropriate action (as outlined in time 60 minutes to 6 hours) above
• Continue IV fluid replacement to achieve positive balance of 3-6 litres by 12 hours
o Maintain an accurate fluid balance chart
• Assess for complications of treatment e.g. fluid overload, cerebral oedema, extra pontine myelinolysis
(e.g. deteriorating conscious level)
• Continue treatment of any underlying precipitant
o If patient not improving seek senior advice
• Avoid hypoglycaemia
o Aim to keep blood glucose 10-15 mmol/L in first 24 hours
o If blood glucose falls below 14 mmol/L commence 5% or 10% glucose at 125 ml/hr AND
CONTINUE 0.9% sodium chloride solution
• Ensure referral has been made to diabetes team
D. 12 to 24 hours
Aim:
• Ensure continuing improvement of clinical and biochemical parameters
o Continue charting blood glucose hourly. Measurement of sodium and calculated osmolality can be reduced to 4 hourly if improvement maintained (if not continue 2 hourly)
o Do not expect biochemistry to have normalised by 24 hr (sodium and osmolality are likely to be raised)
o Take appropriate action (as outlined in time 60 minutes to 6 hours) as outlined above depending on results
• Continue IV fluid replacement to achieve remaining replacement of estimated fluid losses within next 12 hours – this will be dependent on factors such as initial degree of dehydration / body weight etc and MOST IMPORTANTLY the response to treatment so far. Therefore:
o Continue maintaining accurate fluid balance chart, plotting osmolality and make appropriate adjustments to fluid replacement rates
• Continue IV insulin with or without 5 or 10% glucose solution to maintain blood glucose 10-15 mmol/L
o Adjust insulin infusion rate hourly by 1 unit/hr increments or decrements to achieve desired BG
• Assess for complications of treatment e.g. fluid overload, cerebral oedema, extra pontine myelinolysis
(e.g. deteriorating conscious level)
• Continue treatment of any underlying precipitant
o If patient not improving seek senior advice
E. 24 hours to Day 3
Expectation: patient should be steadily recovering, beginning to eat and drink, biochemistry back to normal.
• Ensure that clinical and biochemical parameters are improving or have normalised
o Continue IV fluids until eating and drinking normally
o Variable rate insulin if not eating and drinking
o Convert to appropriate subcutaneous regime when biochemically stable
o Encourage early mobilisation
o Daily urea and electrolytes
o Remove catheter when clinically appropriate
• Assess for signs of fluid overload or cerebral oedema
• Assess for evidence of continuing sepsis
• Daily foot checks
• Continue LMWH until day of discharge (consider extended treatment in very high risk patients)
• Ensure patient has been reviewed by diabetes team
After care
Most patients should go home on subcutaneous insulin (the regime being determined by their circumstances). For patients with previously undiagnosed diabetes or well controlled on oral agents, switching from insulin to the appropriate oral hypoglycaemic agent should be considered after a period of stability (weeks or months). Ensure patient has appropriate diabetes education prior to discharge and arrange follow-up by diabetes team.
Cite This Work
To export a reference to this article please select a referencing stye below: