Introduction
Streptococcus pneumoniae is a gram-positive capsular, bacterial pathogen, which is responsible for many diseases, ranging from fairly harmless (acute otitis media and sinusitis) to deadly (pneumonia, bacteraemia and meningitis). Those effected are the paediatric, elderly and the immunocompromised, with pneumococcal infection being the leading cause of pneumonia in children worldwide. This infection is pandemic, with 23,886 confirmed cases of invasive pneumococcal disease being reported in the EU in 2017 alone (ECDC,2017). Those who are at a greater risk of contracting the disease are individuals with other health conditions such as diabetes, asthma, chronic obstructive pulmonary disease, cardiovascular disease, human immunodeficiency virus (HIV) and sickle cell disease to name but a few (Daniels et al., 2016). Despite it causing disease, S. pneumoniae can colonise the upper respiratory tract asymptomatically.
The bacteria have evolved over time to be able to colonise the respiratory tract of children and healthy adults, which is where it usually resides as a highly adapted commensal (Weisner et al., 2018). They can attach to the nasopharyngeal surface and from here spread to other organs such as the ears and lungs, travel through the blood stream or remain as a coloniser (Normak-Henriques and Tuomanen, 2013). The main role of the capsule is to protect the bacteria from host enzymes, bacteriophages and host immune cells, such as phagocytes. Therefore, acting as a great virulence factor due to its capacity to help the bacterium evade assault from the host immune system (Ford, 2014) and, it is thus frequently a target for vaccine development. Another important feature are the pili, which adorns the cell surface of the bacteriumand is crucial for attachment to epithelial cells (Normak-Henriques and Tuomanen, 2013) – once again acting as a great virulence factor. S. pneumoniae is often transmitted via person-to person contact, usually from asymptomatic carriers (such as children), and requires contact with nasal secretions or contaminated surfaces (Zafar et al., 2017).
Depending on the area of the body that has been infected, the symptoms of S. pneumoniae can vary and often, symptoms cannot be distinguished from other bacterial infections (ECDC, 2014). According to Normak-Henriques and Tuomanen (2013), S. pneumoniae causes more deaths than any other disease globally. It has a plastic genome and can easily and quickly remodel it through uptake and incorporation of external DNA from other pneumococci or from closely related oral streptococci species. Due to its plastic genome, it has been able to become highly resistant to antibiotics such as meropenem, vancomycin, teicoplanin, linezolid and, quinupristin/dalfopristin (Ford, 2014). Its plasticity has thus, allowed it to evade a vaccine-induced immune response. The lethality of S. pneumoniae is therefore as a result of an accumulation of factors such as its virulence factors, a shift from commensal to pathogenic interaction in host, high carriage rates and its genetic plasticity. These are some of the reasons as to why WHO included S. pneumoniae as one of 12 priority pathogens in 2017 (Weisner et al., 2018). Moreover, the above reasons are prime examples as to why vaccines for these bacteria have been created. Additionally, the bacteria have several different serotypes (distinct surface structures) which makes creating vaccines to eradicate the disease as whole more problematic. The 10 most common serotypes for 2017 in the EU were 8, 3, 22F, 19A, 12F, 9N, 15A, 10A, 11A and 23B (ECDC,2017). The number of serotypes used in a vaccine is determined by the prevalence of that serotype and this is why new vaccines are readily being created. The purpose of this essay is to outline some the main treatments for S. pneumoniae and to compare two vaccination treatments – one glycoconjugate vaccine and one whole cell vaccine.
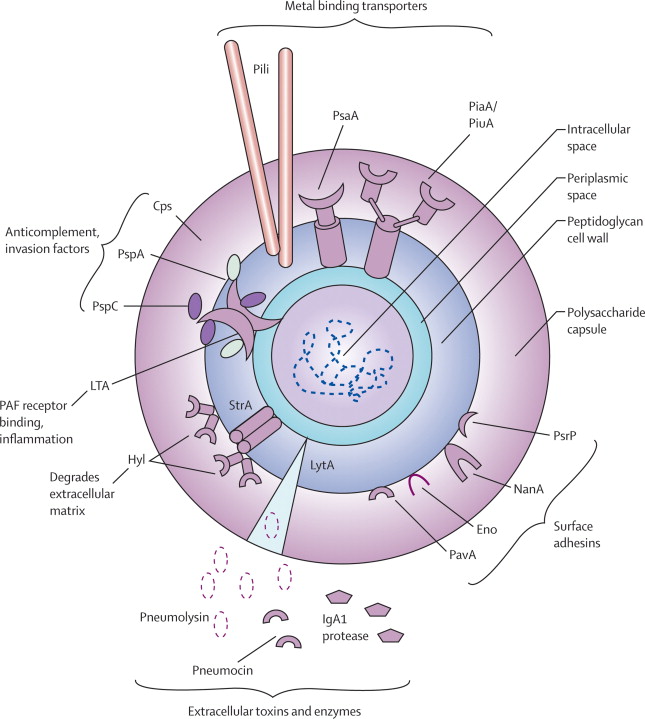
Figure 1. Picture taken from Van der Poll and Opal (2016). Diagram of the Streptococcus pneumoniae bacterium. The polysaccharide capsule depicted, is one of the main targets for vaccine development due to the fact that the capsule is one of the main virulence factors that enables the bacteria to evade host immune response. The pili, which are illustrated, allows for the bacterium to attach to host cell surfaces to commence cell assault. Pneumococcal serine-rich repeat protein (PsrP) is also shown and it mediates attachment to the lung epithelial cells and is encoded by a pathogenicity island.
Treatments
The mainstay treatment for individuals with S. pneumoniae is usually antibiotic therapy – specifically amoxicillin in less severe cases. Oral amoxicillin should be used as the first line of response in the management of community acquired pneumonia in the UK – guidelines set by National Institute for Clinical Excellence (NICE) and British Thoracic Society (BTS). Amoxicillin should be taken from between five and seven days, solely depending on the stage of illness. For those individuals that present with suspected pneumococcal or bacterial meningitis, NICE recommends intravenous cefotaxime alongside amoxicillin or ampicillin. Once further diagnosis has been carried out, such as taking a culture sample from cerebrospinal fluid (CSF), the antimicrobial treatment should be tailored further once the causative agent has been confirmed. The British Infection Association (BIA) recommended penicillin-based agents and cephalosporins in the treatment of community-acquired pneumonia. However, if a patient has highly resistant S. pneumoniae, ofloxacin and vancomycin have been suggested to use. (Brown et al., 2017).
As aforementioned, antibiotic resistance for S. pneumoniae is a cause for concern. However, amoxicillin was created by adding an amino group onto penicillin to combat antibiotic resistance. The mechanism of amoxicillin is as follows: due to it being a beta lactam, it has the ability to bind to penicillin-binding proteins that inhibits a process called transpeptidation. This then results in the activation of autolytic enzymes in the bacterial cell wall. This process leads to lysis of the cell wall and thus bacterial cell death. Another way of combatting antibiotic resistance to amoxicillin is by using amoxicillin and a beta lactamase inhibitor in conjunction e.g. clavulanic acid. They work by “binding irreversibly to the catalytic site of an organisms’ penicillinase enzyme, which causes resistance to the original beta lactam ring. Although this doesn’t directly cause bacterial cell death, when used with amoxicillin, they allow for amoxicillin to perform correctly on those organisms that produce penicillinase activity (Akhavan and Vijhani, 2019).
Apart from solely antibiotic therapy, there are other ways of treating pneumococcal infections such as adjuvant anti-inflammatory therapy. The role of which is to enhance the body’s immune response to the antibiotics. The first example of an adjuvant are macrolides which have both anti-inflammatory and antimicrobial activities – targeting the pathogen and host immune cells. They target the pathogen by having selective inhibitory effects on the protein synthesis of bacterial cells and by suppressing the production of pneumococcal adhesins, invasins and cytotoxins. For the anti-inflammatory activity, the macrolides target neutrophil-mediated inflammation by suppressing the synthesis of neutrophil-mobilising chemokines and cytokines (e.g. tumour necrosis factor), thus inhibiting neutrophil influx. Another adjuvant are corticosteroids, which are “broad-spectrum anti-inflammatory agents”, most commonly used for penicillin-susceptible meningitis. Corticosteroids work by reducing the inflammation caused by the bacterial assault. Another crucial adjuvant are statins, which once again are anti-inflammatory agents. They work by interfering with G-protein receptor-mediated signalling in immune cells. They also activate the enzyme heme oxygenase-1, which mediates synthesis of essential anti-inflammatory proteins (Feldman and Anderson, 2014).
Using antibiotic therapy as form of treatment is a beneficial thing to do as it can slow down the growth of the bacteria, thereby reducing the damaging effects on the body. Additionally, they are fast acting and can begin to work within a few hours of beginning the course of treatment. However, antibiotic resistance can ensue if the antibiotics are consumed regularly, hence reducing the beneficial effect. Also, longer courses of treatments can have a damaging effect to the body’s immune system and may also cause damage to the digestive tract. Nonetheless, as mentioned earlier, there are ways to overcome this issue by using anti-inflammatory adjuvants. Vaccines can be used as a preventative measure against acquiring infectious diseases and is often looked to as a course of action for eradicating pneumococcal diseases – amongst many other types.
Glycoconjugate Vaccine
Glycoconjugate vaccines are carbohydrate antigens chemically linked to a protein. The covalent linkage allows for bacterial surface carbohydrates to evoke a long-lasting T cell memory response, which is accompanied by polysaccharide-specific B cell differentiation into plasma cells. Should the immune system come across the pathogen again, plasma cells will proliferate and release mature antibodies that can eliminate the disease in the immunised individual (Berti and Adamo, 2018). The aim of the study carried out by Kaplonek et al. (2018), was to improve on “the already licensed vaccine Prevnar13 (13-valent) and Synflorix (10-valent) by adding synthetic glycoconjugates representing serotypes that are not covered by existing vaccines and to create a pentavalent semisynthetic glycoconjugate vaccine (sPCV5)” (Kaplonek et al., 2018). Synflorix is a pneumococcal vaccine that contains 10 of the common most serotypes of S. pneumoniae, whereas Prevnar 13 is also a pneumococcal vaccine but has 13 of the most common serotypes of S. pneumoniae and can be used on all ages. In the study, the oligosaccharide antigens were conjugated to CRM197 (a non-toxic variant of diphtheria toxin isolated from Corynebacterium diphtheriae). There were eleven groups of 10- to 12-week old female New Zealand White (NZW) rabbits for the immunisation experiment, with about 3-6 rabbits per group. The control groups were as follows: group one had a combination of all five synthetic glycoconjugates (sPCV5), group two had a combination of Prevnar13 ( serotype antigens ST2 and ST8) and Synfloxrix (serotype antigens ST2, ST3 and ST8) with non-vaccine serotypes, group three had Prevnar13 only, group four had Synflorix only and group five had a placebo (CRM197 and 125µg of aluminium). Each rabbit received four doses of vaccine in a final volume of 0.5mL. On day 0, the rabbits were immunised and boosted with the same vaccine on days 14, 28 and 119. The rabbits were bled at days 0, 14, 21, 35, 119 and 126 for their sera. These antibody responses were then analysed using ELISA and glycan microarray.
The results obtained from the ELISA and glycan microarray showed that the rabbits that had been immunised with the glycoconjugates induced strong anti-glycan antibody titres. Additionally, the co-formulated vaccines (Prevnar and Synflorix) elicited strong immune responses but were weaker than those observed with immunisation from only one serotype – this is due to a weaker serotype-specific antibody response. sPCV5 elicited a stronger polysaccharide-specific antibody response compared to Prevnar and Synflorix (the marketed vaccines). Overall, the serum dilutions found, that are necessary for killing 50% of the bacteria, are considered “biologically significant” for industry standard of a successful vaccine.
The 23-valent polysaccharide is not effective in infants and therefore, having a pentavalent vaccine that is able to be used on all age groups is beneficial, especially with the added serotypes covered to broaden the immune capabilities. Co-formulating vaccines is not as efficacious because it produces a weakened immune response compared to those with a single vaccine. This could possibly be due to the increased amount of serotypes involved, which means the immune system would have to deal with all of those serotypes thus, causing a slower immune response. This could also be said for adding serotypes to the pentavalent vaccine if they wanted to increase the amount in the future to cover more serotypes. Although this study had efficacious results, it can be argued that the results may not be applicable as the test subjects were rabbits. Rabbits may have a different immune response than humans and the vaccines may work better or worse in the rabbits compared with the humans – only when the tests are carried out on humans can it be fully applicable. Moreover, the paper does not appear to have any thorough statistical analysis to compare results from each control group in the study, therefore reducing the validity of the results as comparison between groups is hard to be done without statistical analysis.
Whole Cell Vaccine
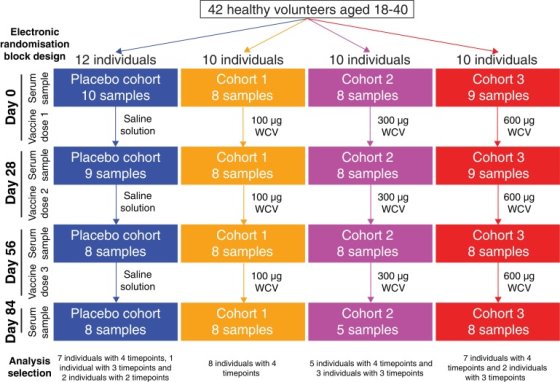
The next vaccine to be discussed, conducted by Campo et al. (2019), uses the whole bacterium of Streptococcus pneumoniae in the vaccine, referred to as whole cell vaccination (WCVs). As the name suggests, whole cell vaccines use the entirety of the cell to produce a vaccine and thus an immune response resulting in immunity. The aim of this study is to produce a vaccine that is able to be used in developing countries where it may be too expensive to get vaccinated. Using a whole cell vaccine should be more economical for those who wish to get vaccinated. 42 healthy U.S adults aged 18-40 participated, using a randomised double-blind trial where four cohorts were compared (the pharmacists who were injecting the vaccine or placebo were not blinded). The participants had no sign of chronic health conditions or history of pneumococcal disease or infection. Of the four, one cohort received a placebo of saline injections, the second cohort received 100µg of WCV, the third 300µg of WCV and the fourth received 600µg of WCV, all of which were absorbed to aluminium hydroxide adjuvants. Every 28 days, each participant was given three injections and their serum samples were taken pre-vaccination. At 28, 56 and 84 days, their serums were taken, following the first injection to assay for immunoglobulin G (IgG) responses (day 0) – as referred to in figure 2. The samples were then analysed with a panproteome microarray which included more than 2100 probes. The purpose of using the panproteome microarray is that it provides information on responses to proteins encoded by the core and accessory genome as well as variants of diverse core loci (DCL), which corresponds to genes that can be identified in almost all isolates based on their location in the chromosome. In S. pneumoniae, there are four loci that encodes for important pneumococcal surface proteins.
Flow Chart Depicting the Sample Collection of Data
Figure 2. Flow chart taken from Campo et al., (2018) depicting the sample collection of data for the trial. 12 individuals constituted the placebo trial with the first sample of serum being taken on day 0 before vaccine dose. 10 individuals constituted the 100µg, the 300µg and the 600µg cohorts being vaccinated with the WCV, with each of them having their serum levels taken on day 0 before the vaccine dose. Each individual’s in the cohorts had differing time points.
130 samples were studied from 35 of the 42 trial participants, for 27 individuals, all of the four timepoints were analysed. At least the initial and final samples were available for two further people and at least one timepoint was analysed for six more people. Of these individuals, 35 of the them were the placebo, 32 from cohort 1 (100μg dose), 29 from cohort 2 (300μg dose) and 34 from cohort 3 (600μg dose). For the samples, there were no biological repeats done as they had already done a pilot study which showed that there were reproducible differences between the individuals. A Kruskal-Wallis test was carried out that showed no significant difference in individuals’ median IgG binding to S. pneumoniae proteins between cohorts before vaccination. This indicates that the WCV did not raise IgG binding to a high proportion of S. pneumoniae proteins across all of the cohorts. However, an ANOVA test was carried out for the change from day 0 to day 84 and a significant difference between the cohorts was found. Therefore, showing that the WCV did have an effect on the vaccinated cohorts when considering per-probe, and not per-individual data. The difference in results showed could be as a result of individual differences in the subsets.
The trial used differing concentrations of the WCV which is good as it enables a comparison to be made between the different concentrations, and allows for the right dosage of drug to be formulated for public use. The idea of using a whole cell vaccine has great ethical implications as it is said to be more cost-effective and enables those from poor incomes such as third world countries to be able to have access to the vaccine. The trials were carried out on humans which means that the study is more applicable as the results from the trials will have more validity as the immune responses from the trials are human responses and thus have real world relatability. The results from this trial could be in-part due to anamnestic responses received from previously recognised antigens, which may have caused the individual’s immune response to be limited to the WCV-induced IgG response. In addition, not all individuals in each cohort had their serum collected and tested for the four timepoints of day 0, day 28, day 56 and day 84. This reduces the reliability of the study as not only were there no biological repeats, but the results were lacking in a large enough sample size for each cohort to make the results more valid. In addition, the overall sample size was lacking as there were not enough participants to see whether there would be an effect of the vaccine. This is because there could be confounding variables such as whether the participants were male or female (not stated in the study) and participants were all from America so things like diet and ethnic background also need to be taken into account.
Conclusion
In conclusion, both vaccines found efficacy in their trials, however the whole cell vaccine is more applicable as it was done on humans and their immune response, as opposed to the glycoconjugate vaccine trial which was carried out on rabbits. On the other hand, it can be argued that the data collected for the trial was inadequate to make a fair comparison as not all of the participants’ serum was tested for all four of the timepoints (every 28 days). Despite that, the vaccine has real world application. For example, the vaccine is said to be more economical and therefore can hopefully be used in countries that are impoverished, and for those individuals who are unable to afford the more expensive vaccines. This is beneficial as it means that more people should be able to get vaccinated and the risk of acquiring the disease should become lower. This is also beneficial for issues such as antibiotic resistance, as herd immunity will mean that less individuals will be susceptible to getting the disease as the immune system would be primed to fight off the infection if/or when it enters the body. Therefore, antibiotic resistance to these bacteria would be of less importance as the majority of individuals would be immune, providing that the most prevalent serotypes are vaccinated against e.g. by using a similar technique to that of Kaplonek et al. (2018). Important questions to be asked about the whole cell vaccine is whether it would require more than one dose – if so, would it still be the cheaper option for those from poorer backgrounds?
Bibliography
- Akhavan, B. and Vijhani, P. (2019). Amoxicillin. StatPearls, [online]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK482250/ [Accessed 15th Oct. 2019].
- Berti, F. and Adamo, R. (2018) Antimicrobial glycoconjugate vaccines: an overview of classic and modern approaches for protein modification. Chemical Society Reviews, [online], volume 47(24), pp. 9015-9025. Available at: https://pubs.rsc.org/en/journals/journalissues/cs#!recentarticles&adv [Accessed 26th Oct. 2019].
- Brown, L., Mitchell, A. and Mitchell, T. (2017). Streptococcus pneumoniae and lytic antibiotic therapy: are we adding insult to injury during invasive pneumococcal disease and sepsis? Journal of Medical Microbiology, volume 66(8), pp. 240-255. Available at: https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.000545 [Accessed 15th Oct. 2019].
- Campo, J., Le, T., Pablo, J., Hung, C., Teng, A., Tettelin, H., Tate, A., Hanage, W., Alderson, M., Liang, X., Malley, R., Lipsitch, M. and Croucher, N. (2018). Panproteome-wide analysis of antibody responses to whole cell pneumococcal vaccination. eLife, [online], volume 7(1), pp. 336-410. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6344088/ [Accessed 15 Oct. 2019].
- Daniels, C., Rogers, P.D. and Shelton, C. (2016). A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. The Journal of Pediatric Pharmacology and Therapeutics, [online], volume 21(1), pp. 27-35. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4778694/ [Accessed 15th Oct. 2019].
- European Centre for Disease Prevention and Control (2014). Disease fact sheet about pneumococcal disease, [online]. Available at: https://www.ecdc.europa.eu/en/pneumococcal-disease/facts [Accessed 26th Oct. 2019].
- European Centre for Disease Prevention and Control (2017). Invasive pneumococcal disease: annual epidemiological report for 2017. [Online] Available at: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-invasive-pneumococcal-disease.pdf [Accessed 26th Oct. 2019]
- Feldman, C. and Anderson, A. (2014). Recent advances in our understanding of Streptococcus pneumoniae infection. F100 Prime Reports, [online], volume 6, pp. 86-90. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4166932/, [Accessed 15th Oct. 2019].
- Ford, M. (2014). Medical Microbiology. 2nd ed. Hampshire: Oxford, p. 4.
- Kaplonek, P., Khan, N., Reppe, K., Schumann, B., Emmadi, M., Libosa, M., Xu, F., Calow, A., Parameswarappa, S., Witzenrath, M., Pereira, C. and Seeberger, P. (2018). Improving vaccines against Streptococcus pneumoniae using synthetic glycans. Proceeding of the National Academy of Sciences of the United States of America, [online], volume 115(52), pp. 13353-13358. Available at: https://www.pnas.org/content/115/52/13353 [Accessed 15th Oct. 2019].
- Normak-Henriques, B. and Tuomanen, E. (2013). The Pneumococcus: Epidemiology, Microbiology, and Pathogenesis. Cold Spring Harbor Perspectives in Medicine, [online], volume 3(7), p. 10215. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3685878/ [Accessed 13th Oct. 2019].
- Shivshankar, P., Sanchez, C., Rose, L. and Orihuela, C. (2009). The Streptococcus pneumoniae adhesin PsrP binds to Keratin 10 on lung cells. Molecular Microbiology, [online], volume 73(4), pp. 663-679. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2753542/ [Accessed 26th Oct. 2019].
- Van der Poll, T. and Opal, S. (2009). Pathogenesis, treatment, and prevention of pneumococcal pneumonia. The Lancet, [online], volume 374(97), pp. 1543-1556. Available at: https://www.sciencedirect.com/science/article/pii/S0140673609611144 [Accessed 26th Oct. 2019].
- Weisner, J., Ferreria, D. and Paton, J.C. (2018). Streptococcus pneumoniae: transmission, colonisation and invasion. Nature Review Microbiology, [online], volume 16(6), pp. 355-367. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5949087/ [Accessed 15th Oct. 2019].
- Zafar, M.A., Wang, Y., Hamaguchi, S. and Weiser, J.N. (2017). Host-to-host transmission of Streptococcus pneumoniae is driven by its inflammatory toxin, pneumolysin. Cell Host and Microbe [online], volume 21(1), pp. 73-83. Available at: https://www.sciencedirect.com/science/article/pii/S1931312816305169 [Accessed 20th Oct. 2019].
Cite This Work
To export a reference to this article please select a referencing style below:
Related Content
All TagsContent relating to: "pneumonia"
Pneumonia is an inflammatory condition of the lung which can result from infection with particular bacteria, viruses or other organisms. It is characterised by lung parenchyma inflammation and the filling of the air-filled sacs of the lung (alveoli) with fluid resulting in a decrease in elasticity which leads to inefficient gas exchange.
Related Articles