Discuss how dietary modification represents a platform for the prevention of gastrointestinal cancer
Abstract
Gastric cancer provides a good alternative to show the association of pathogenesis in the gastrointestinal tract and dietary modification. High salt intake and n-nitroso compounds have been identified in having a very strong relationship in the promotion of gastric cancer. The synergy between Helicobacter pylori and these dietary modifications elevates the chances of gastric cancer. Salt consumption leads to the inflammation of the stomach lining and it increases the colonisation of Helicobacter pylori which can cause the upregulation of iNos, COX-2 and CagA which cause increase the risk of gastric cancer. Similarly, H.pylori increases the chances of the formation of the carcinogenic n-nitroso compounds via increase nitrosation. Also, the reduction of Vitamin C, which acts as a free radical scavenger causes an increase in nitrosation which can ultimately increase gastric cancer. Ultimately, dietary modifications do play an integral role in the pathogenesis in gastrointestinal cancer.
Introduction
Absorption, motility and digestion are some of the major physiological processes which take place in the gastrointestinal tract. Therefore, the association modification of diet may play a significant role in the pathogenesis of gastrointestinal cancer. The gastrointestinal tract starts from the alimentary canal in the mouth to the anus provides a vast opportunity to explore the relationship between dietary modification and different types of cancers. However, focusing on one specific cancer allows the identification of the depth in which dietary modification can provide a stable preventive mechanism.
Gastric cancer is the fifth most common cancer in the world. (1) It is the third most common cause of cancer mortality due to the overall late symptoms being identified and therefore, lower prognosis.(1) Therefore it is imperative that dietary modification is implemented so that there is early prevention of gastric cancer and a higher life expectancy. Helicobacter pylori (H. pylori) is a known carcinogenic, which increased the risk of gastric cancer via chronic inflammation. (2) H. pylori situates in the stomach and this is a strong link between diet and gastric cancer as it can directly affect the microenvironment of the stomach.
- Salt
Sodium is one of the most important electrolytes in the modulation of plasma osmolality and body fluid volume. (3) The regulation of sodium in the body was much easier 5000 years ago, as the dietary sodium levels were much lower than modern times; Salt is now more exposed in our diet and it is often used in preservation techniques. (4) understanding some of the mechanism in which salt is used in the development in food may allow the association between gastric cancer and salt to be identified; high sodium concentrations in the stomach has been associated with inflammation and mucosal damage; this is turn may cause an increase in mutation and increase proliferation of cells leading to gastric cancer. REF
- Mechanisms
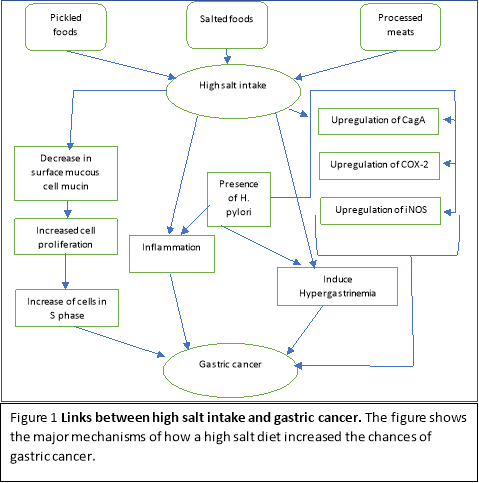
There are many mechanisms which show the link between salt intake and gastric cancer. Infection of bacteria like H. pylori and high dietary salt intake can cause inflammation, leading to a higher rate of colonisation of H. pylori. (5) The high salt intake caused a decrease in gland mucous cell mucin. (6) A rat study showed that high dietary salt intake leads to a reduction in the cell yield and an increase in cells which were in the S phase. This increases the cancer risk as it increases the chances of mutations and therefore formation of gastric cancer. (7) In gerbils, in the presence of H. pylori and high dietary salt, the upregulation of iNOS and COX-2. (8) This upregulation has been theorised to enhance the effect of H. pylori and subsequently promote the pathogenesis of gastric cancer. (9) In addition, high salt intake also leads to the upregulation of CagA which enables the gene to be able to promote H.pylori with the ability to alter the function of the parietal cells.(10) High salt intake can also consequently induce hypergastrinemia. (6) The combination of H. pylori and hypergastrinemia may lead to the progression of gastric cancer as it may contribute to a decrease in parietal cells.
Humans contain two types of gastric mucins: surface mucous cell mucin (SMCM) and gland mucous cell mucin (GMCM).(11) A study by Hidaka et al (12), showed that H.pylori only attached to the surface mucous epithelial cells. The penetration of only one type of gastric mucin is due to the upregulation of the GMCM against the H.pylori infection. (13) This displays that there are two mechanisms in which salt can potentially increase the risk of gastric cancer. A direct mechanism is the increase in cell proliferation due to the mucosal injury by the H. pylori. The indirect mechanism is to provide support in the immediate environment of the stomach to increase colonization of H. pylori. A study by Furihata et al (14) showed that there was damage to the gastric tissue when hypotonic NaCl solution was administered. Although the damage was temporarily and the tissue was back to its’ original state within one-two days, it is important to consider that prolonged exposure to high salt intake will cause excessive damage and therefore increased the risk of carcinogenesis in the gastric tissue.
- Epidemiological & case cohort studies
Kato et al. (6) demonstrates that salt does support gastric malignancies in a dose-dependent factor when H. pylori is present. Although this result was achieved in an animal study, this relationship can be used to express the idea that a reduction in salt and salty food can decrease gastric cancer in humans. Therefore, considering a study by Tsugane et al. (15) up to 12% of salt is consumed in dietary sources such as pickled vegetables, preserved fish and salted fish. The synergistic behaviour of salt with H. pylori exposes the importance in the prevention of a high dietary salt intake to prevent the onset of gastric cancer.
D’Elia et al (16) showed that moderately-high and high salt intake increased the chances of gastric cancer by 41% and 68% respectively, when compared to low salt intake. Japanese individuals who were habitually inclined to consume salt-rich foods had a higher risk of gastric cancer; with the consumption of pickled foods, salted fish and processed meats, there was an increased risk of 27% , 24% and 24% respectively. (16)
A study showed that the association with individuals having a gastric cancer screening is lower in individuals with higher salt intakes.(17) Therefore, in this epidemiology study, it can be identified that the Korean population may not be aware of the consequences of a high salt diet; Therefore, a better delivery in the consequences of dietary salt intake may lead to more gastric cancer screening and potentially show an indirect decrease in the rate of gastric cancers in the population.
- N-nitroso compounds
N-nitroso compounds (nocs) are found both in exogenous sources as well as endogenous synthesis in the body; exogenous synthesis is based upon the consumption of food sources such as processed meat and pickled vegetables. (18) The endogenous synthesis of nocs take place via nitrosation of amides by nitrite based nitrosation gents. (19) The endogenous synthesis is potentially caused by the haem group in red meat. (20) Nocs have been found to be carcinogenic (18); this allows us to have an insight into mechanisms in which carcinogenic compounds may interact in the stomach and cause gastric cancer.
- Mechanisms
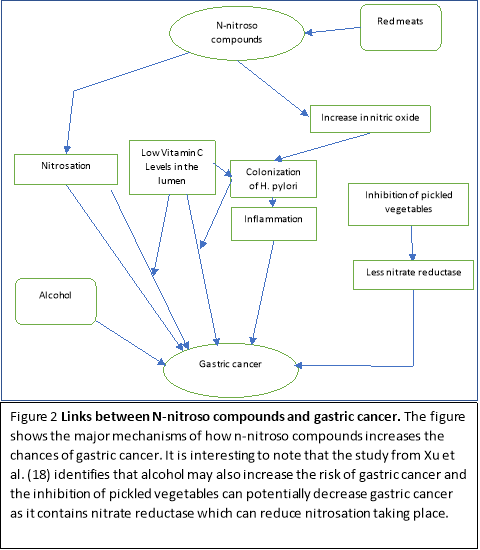
The mechanisms which link nocs and nitration described allows a powerful insight into some of the potential processes that are modulated in the prognosis and progression of gastric cancer; this allows us to identify the food sources that may potentiate the advancement of gastric cancer and therefore allow dietary modification to prevent gastric cancer. There are several mechanisms which allow nitrosation and consequently the formation of nocs to take place. Firstly, nitrosation is more likely to take place in a more acidic stomach. (21) There is also synergy of nitrosation at low vitamin C levels. (21) Secondly, inflammatory conditions are developed in the stomach in the presence of H. pylori when nitrosation takes place. (22) Lastly, Individuals who are exposed to high concentrations of nocs have a higher potential risk in acquiring gastric cancer (18).
- Epidemiological & case cohort studies
A study by Jakszyn et al. (23) has shown an increase in faecal nocs with the consumption of red meat. This has been observed by the reaction involving haemoglobin and myoglobin which reacts with nitric oxide to produce nocs which exist in the upper gastrointestinal tract. The results show that H. pylori infection increases nitric oxide due to the bacterial response being counteracted by macrophages; this will in effect cause an increase in nitrosation due to the high nitric oxide concentrations and subsequently allow high colonisation of h pylori. The red meat therefore does increase the risk of gastric cancer and this is also supported by other studies. (24) Having high levels of vitamin C may potentially prevent the onset of gastric cancer as it acts as a free radical scavenger and it enhances the mucosal formation; This reduces the synthesis of nocs and inhibits the optimum environment for H. pylori reducing cell proliferation of H. pylori. (25) The H. pylori counteracts this by causing inflammation of the stomach, causing a decrease of in the secretion of vitamin C in the lumen. (25) This exemplifies the synergistic relationship between reduced vitamin C levels and H. pylori in causing gastric acid; hence an increase in vitamin C should theoretically reduce the colonisation of H. pylori as well as the formation of nocs.
A study by Xu et al. (18) shows the association between gastric cancer and nocs accurately using cofactors from endogenous nitrosation. The precursors of nocs such as nitrite, nitrate and 4 nocs were measured in urine. Firstly, the results showed a strong significant positive correlation with urinary nitrate and the risk of gastric cancer risk with negative igG antibodies for H. pylori. (18) Secondly, there were also increased levels of nitrite with the presence of H. pylori. (18) Finally, it also showed a link between alcohol consumption and some specific nocs. (18) The results highlight that there is a direct relationship between the formation of nocs and pathogenesis of gastric cancer. The addition of H. pylori synergistically allows the advancement of nitrosation and formation of nocs in the stomach. It also showed that decreased alcohol consumption may lead to a decrease in the formation of gastric cancer as there would be less carcinogenic nocs forming. Interestingly, this study has also shown a significantly inverse association with pickled vegetables and urinary nitrate levels. (18) Pickled vegetables contain nitrate reductase which can reduce nitrate to nitrite; Thereby causing a reduction in the nitrosation process, leading to less nocs synthesised. (18) Ultimately, this study shows the significant relationship between exogenous dietary sources such as red meat, alcohol should be limited to prevent gastric cancer.
Conclusion
The association between dietary modification and the prevention of gastric cancer is very high. Avoiding processed meat such as red meat which are high in n-nitroso compounds and food sources which are high in dietary salt can prevent damage to the mucosal lining of the stomach and prevent mutations. Interestingly, pickled vegetables have specifically shown many implications in the prevention of gastric cancer. Although, pickled vegetables do have a high salt intake and therefore would increase the risk of gastric cancer by inflammation of the mucosal lining, it does contain a bacteria called nitrate reductase which is able to reduce the processes of nitrosation, enabling less carcinogenic n-nitroso compounds to form and therefore, a decrease the number of mutagenic events in the stomach. This highlights variation of dietary modification as they may play a different role in different mechanistic pathways in the gastrointestinal tract. Dietary modification consequently can provide a strong supportive role in the prevention of gastrointestinal cancer by the effects seen in the microcosm of the stomach.
References
1. World Cancer Research Fund / American Institute for Cancer Research. Diet, nutrition, physical activity and stomach cancer. 2016;
2. Sepulveda AR. Helicobacter, Inflammation, and Gastric Cancer. Curr Pathobiol Rep. 2013 Mar 2;1(1):9-18.
3. Antunes-Rodrigues JJ, de Castro M, Elias LLK, Valenca MM, McCann SM, Valença MM, et al. Neuroendocrine control of body fluid metabolism. Physiol Rev. 2004;84(1):169-208.
4. Cordain L, Eaton SB, Miller JB, Mann N, Hill K. The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002 Mar;56 Suppl 1:S42-52.
5. Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59(19):4823-8.
6. Kato S, Tsukamoto T, Mizoshita T, Tanaka H, Kumagai T, Ota H, et al. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer. 2006;119(7):1558-66.
7. Charnley G, Tannenbaum SR. Flow cytometric analysis of the effect of sodium chloride on gastric cancer risk in the rat. Cancer Res. 1985;45(11 II):5608-16.
8. Toyoda T, Tsukamoto T, Hirano N, Mizoshita T, Kato S, Takasu S, et al. Synergistic upregulation of inducible nitric oxide synthase and cyclooxygenase-2 in gastric mucosa of mongolian gerbils by a high-salt diet and Helicobacter pylori infection. Histol Histopathol. 2008;23(5):593-9.
9. Nozaki K, Shimizu N, Inada K, Tsukamoto T, Inoue M, Kumagai T, et al. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn J Cancer Res. 2002 Oct;93(10):1083-9.
10. Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007 May 15;67(10):4709-15.
11. Ota H, Katsuyama T. Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem J. 1992;24(2):86-92.
12. Hidaka E, Ota H, Hidaka H, Hayama M, Matsuzawa K, Akamatsu T, et al. Helicobacter pylori and two ultrastructurally distinct layers of gastric mucous cell mucins in the surface mucous gel layer. Gut. 2001;49:474-80.
13. Matsuzwa M, Ota H, Hayama M, Zhang MX, Sano K, Honda T, et al. Helicobacter pylori infection up-regulates gland mucous cell-type mucins in gastric pyloric mucosa. Helicobacter. 2003 Dec;8(6):594-600.
14. Furihata C, Ohta H, Katsuyama T. Cause and effect between concentration-dependent tissue damage and temporary cell proliferation in rat stomach mucosa by NaCl, a stomach tumor promoter. Carcinogenesis. 1996;17(3):401-6.
15. Tsugane S, Sasazuki S, Kobayashi M, Sasaki S. Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer. 2004;90(1):128-34.
16. D’Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: A meta-analysis of prospective studies. Clin Nutr. 2012;31(4):489-98.
17. Shin JY, Kim J, Choi KS, Suh M, Park B, Jun JK. Relationship between salt preference and gastric cancer screening: An analysis of a nationwide survey in Korea. Cancer Res Treat. 2016;48(3):1037-44.
18. Xu L, Qu YH, Chu X Di, Wang R, Nelson HH, Gao YT, et al. Urinary levels of N-nitroso compounds in relation to risk of gastric cancer: Findings from the Shanghai cohort study. PLoS One. 2015;10(2):1-16.
19. Calmels S, Ohshima H, Rosenkranz H, McCoy E, Bartsch H. Biochemical studies on the catalysis of nitrosation by bacteria. Carcinogenesis. 1987 Aug;8(8):1085-8.
20. Lunn JC, Kuhnle G, Mai V, Frankenfeld C, Shuker DEG, Glen RC, et al. The effect of haem in red and processed meat on the endogenous formation of N-nitroso compounds in the upper gastrointestinal tract. Carcinogenesis. 2007;28(3):685-90.
21. Suzuki H, Iijima K, Moriya a, Mcelroy K, Scobie G, Fyfe V, et al. Conditions for acid catalysed luminal nitrosation are maximal at the gastric cardia. Gut. 2003;52(8):1095-101.
22. Mirvish SS. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995;93(1):17-48.
23. Jakszyn P, Bingham S, Pera G, Agudo A, Luben R, Welch A, et al. Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis. 2006;27(7):1497-501.
24. González CA, Jakszyn P, Pera G, Agudo A, Bingham S, Palli D, et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006 Mar 1;98(5):345-54.
25. Zhang ZW, Farthing MJ. The roles of vitamin C in Helicobacter pylori associated gastric carcinogenesis. Chin J Dig Dis. 2005;6(2):53-8.
Cite This Work
To export a reference to this article please select a referencing style below:
Related Content
All TagsContent relating to: "cancer"
Cancer comprises several diseases that are characterized by abnormal cell growth. There are over one hundred varieties of cancer usually named for their cell or organ type. Cancer is the root cause of death across the world evidenced by WHO which established that around one in six deaths all over the world are due to cancer.
Related Articles


