Introduction
Most human diseases have a genetic component. For some, a genetic alteration is the primary or direct cause. In this category fall chromosomal conditions, which are due to a change in the number or structure of chromosomes, affecting many genes (e.g., trisomy 21) and monogenic or Mendelian diseases, due to the presence of pathogenic variants in a single gene (e.g., cystic fibrosis).
In other conditions, genetic components play either a predisposing or protective role which, whereby interaction with each other and with environmental factors, give origin to what are known as complex or multifactorial diseases (examples include congenital heart disease, obesity, diabetes, etc.)
It is estimated that approximately 6% of all live births have a serious birth defect. (Christianson, 2006) If this is the case, then diagnostic and treatment interventions (e.g., diet supplementation, antiviral treatments or gene editing techniques) could benefit almost 8 million children worldwide every year (Savulescu, 2015).
An argument used against Birth Defects research is that in vitro fertilization, pre-implantation genetic diagnosis (PGD) or termination of pregnancy can ensure that only healthy children are born: You just need to create multiple embryos, do PGD and select disease-free embryos for implantation. However, the argument does not hold. PGD cannot rule out complex multi-genic diseases or common dispositions, even radically increasing the numbers of embryos a couple produces (Bourne, 2012).
Another flaw in the argument is that it lacks to consider the role of environmental factors and infections acquired in-utero or during the birth process, a significant cause of foetal and neonatal mortality and a known contributor to birth defects. Unfortunately, the affected newborn infant may not show any signs until later in life, making the diagnosis even harder.
In the following essay, I will exemplify how the study of environmental factors that can lead to birth defects has furthered our understanding of normal human embryonic development and of the pathogenesis of congenital anomalies, while it has helped to improve clinical diagnosis, prognosis and the development of preventive and therapeutic strategies.
Environmental factors
Discrete environmental factors that can have an effect over the intrauterine environment and the human embryo development are sometimes referred to as “maternal” factors, to differentiate them from genetic aetiologies. The impact these factors can have over foetal or neonatal morbidity and mortality will depend very much on the specific period of the development in which they present (Moreau, 2019).
Bacteria, viruses and parasites can be transmitted from the mother to the foetus or newborn, what is known as “vertical” transmission, and can cause serious to both mother and child. The acronym “TORCH” (Toxoplasma gondii, other, Rubella virus, Cytomegalovirus and Herpes simplex virus) is still misleadingly been used to refer to a foetus or newborn who presented clinical features compatible with a perinatal infection (Schleiss, 2018).
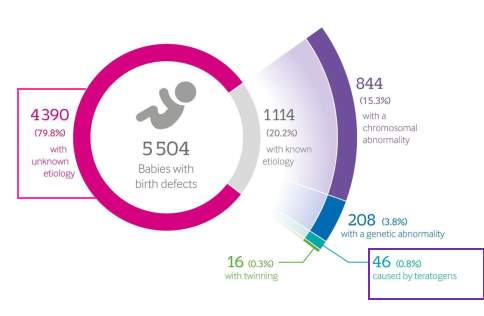
The contribution of infectious diseases and teratogens to birth defects is well recognized (Figure 1.). Hence, many screening recommendations using serology or PCR tests during pregnancy have been published. Less than 1% of birth defects attributed to teratogens seams negligible. However, almost 80% of birth defects still do not have a known aetiology. Congenital infections and teratogens research might shed a light on these cases with non-recognised origin.
It has been shown that prenatal exposures to teratogens and microorganisms can affect the epigenetic machinery (histone modifications, DNA methylation and non-coding RNA expression) responsible for gene and protein expression. These dysregulations can result in foetal death, dysmorphic features or developmental delays. (Aref-Eshghi, 2018).
Figure 1. Known and unknown aetiologies of birth defects. Modified from Marcia L Feldkamp et al. BMJ 2017; 357.
Shepard’s Criteria
Historically, two approaches have been used to identify potential teratogens, defined as “exposures to a mother during pregnancy that have a harmful effect on her embryo or foetus” (Rasmussen, 2011):
a) The ‘rare exposure – rare defect’ combination (also referred to as the “astute clinician approach” (Rasmussen, 2011).
b) Epidemiologic studies data
Congenital Rubella Syndrome and Foetal Alcohol Syndrome are examples of the ‘rare exposure – rare defect’ approach, identified respectively after an ophthalmologist noted cataracts in an infant whose mother had had rubella during pregnancy (Dudgeon, 1967) and after heavy alcohol use during pregnancy was linked to a characteristic pattern of malformations (Hanson, 1978).
On the other hand, Valproic Acid was identified as a teratogen after a case – control study showed an odds ratio of 20 for the association of Spina bifida and the use of Valproic Acid during pregnancy) (Tomson, 2016).
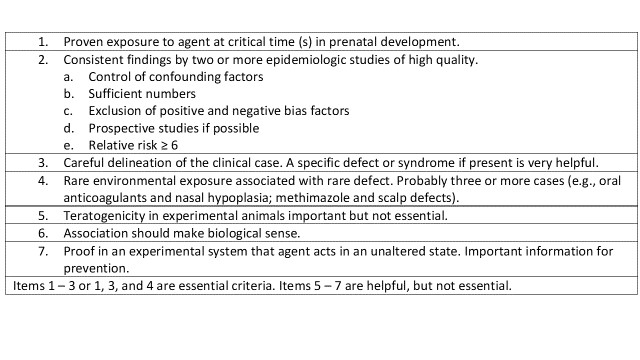
In 1994, a set of seven criteria that incorporated both approaches was proposed for “proof” of teratogenicity (Table 1.). These criteria are still in use to guide teratology – related litigations and to assess new potential teratogens (Schachtman, 2016).
Table 1. Criteria for Proof of Human Teratogenicity. Adapted from Shepard, T.H. (1994) “Proof” of human Teratogenicity. Teratology 50: 97-8.
 Understanding human embryonic development: Organoids to study teratogens.
Understanding human embryonic development: Organoids to study teratogens.
The study of compounds that can cause foetal toxicity have provided valuable information related to embryogenesis and pushed forward the development of new technologies. Specifically, the technical and ethical problems that apply to human development modelling led to the creation of organoids (3D organ-specific models).
The recent Zika virus outbreak dramatically increased the prevalence in microcephaly, particularly in Latin America (Wheeler, 2018). Since the outbreak, many groups have used neural organoids to examine the effect of the Zika virus on neural development. To evaluate the differences of the organoids to in vivo neural tissues required the assessment of organoid size (Cugola, 2016), apoptosis markers (Cui, 2016), immunostaining (Xu, 2016) and transcriptional analysis (Di Lullo, 2017).
Figure 2. Cortical alterations in human brain organoids infected with ZIKV. From Cugola et al. Nature 534, 267–271 (2016)

In the process, human iPSCs were shown to be able to generate motor and glutamatergic neuron-specific organoids (Farkhondeh, 2019). At the same time discrepancies in the organoids assessment results led to the acknowledgement of hESC derived cell types pertinent to neural development (Srinivasan, 2019).
Teratogenicity studies have also contributed to the progress in heart, kidney and retinal organoids development. Cardiomyocytes derived from hPSCs have been used to evaluate the effect of different drugs on contractibility and to visualize markers by immunostaining (e.g., MLC2v, Ki-67, and a-actinin) (Friedman, 2017). Using organoids as a testing platform helped to ascertain that the metabolic switch to fatty acid oxidation that happens in vivo when using palmitate “facilitated metabolic, transcriptional and cell cycle maturation” (Mills, 2017).
Organoids allow evaluating and mimicking the later stages of development. It is pending yet the evaluation of a) the accuracy of these systems in mirroring organ development, b) the uniformity in organoid formation, relevant for high-throughput applications and c) performance of multiple organoids simultaneously (Nio, 2019).
Unfortunately, the most damaging effects of infections and teratogenic compounds are seen when the exposure occurs early during the first trimester of pregnancy. Since organoids do not adequately replicate early embryonic events, more recent studies have looked at culturing human embryos and have observed, among other thing, human amniogenesis when using hPSCs (Shao, 2017).
Embryo cultures brings up ethical questions, since toxicity studies require a large number of human embryos and the current 14-day rule gives no space for long-term studies.
Understanding the pathogenesis of congenital anomalies: Valproic acid (VPA)
VPA, a commonly used anticonvulsant drug, can cause major congenital anomalies in as much as 10% among the offsprings of women treated with the drug during pregnancy (Ornoy, 2017). The teratogenic mechanisms of action of VPA are multiple: Folic acid depletion, arene oxide toxic intermediates, enhanced oxidative stress and induction of gene expression changes (Volmar, 2015).
Folic acid deficiency may trigger changes in gene expression and protein synthesis and can increase oxidative stress (Ornoy, 2018). These changes can explain the wide range of congenital anomalies, including neural tube defects (NTD) and neurodevelopmental delay. VPA treatment in mice was used to show that during organogenesis changes in folate metabolism induced NTD (Akimova, 2017) and that homocysteine augments VPA induced exencephaly (Cabrera, 2018).
VPA can reduce Superoxide Dismutase (SOD) activity and glutathione levels (Ornoy, 2019a). It is suspected that VPA – induced oxidative stress is related to nitrous oxide (NO) production (Fathe, 2014). Sildenafil citrate, which inhibits the 5-phosphodiesterase that increases the action of NO, was found to reduce the rate of VPA – induced NTD in foetal mice (Tiboni, 2015).
Antiepileptic drugs like VPA generate toxic intermediates when metabolised by cytochrome P450 (Johannessen, 2010). Arene oxide, one of the toxic intermediates, can bind to macromolecules and damage embryonic cells. Epoxide hydrolase inactivates arene oxide. Therefore, a lower activity of the enzyme leads to higher concentrations of arene oxide, damaging the developing embryo and foetus (Kelly, 2018).
VPA can inhibit histone deacetylase (Göttlicher, 2001). Both, up and down regulation of gene expression has been described after VPA induced DNA methylation changes. Again, this can also explain the wide range of VPA-induced congenital anomalies, including an increase in autism rate (Eshraghi, 2018). Table 2 shows different developmental and regulation molecular pathways affected by VPA and Carbamazepine (CBZ), another antiepileptic drug.
Table 2. Pathways significantly enriched after VPA exposure and/or after CBZ exposure, involved in neurogenesis or pharmaceutical mechanism of action, showing the total number of genes described within the pathway and the number statistically significantly regulated after exposure. From Schulpen, Pennings, and Piersma, Toxicological Sciences 146 (2): 311–320 (2015).

Contributions to clinical diagnosis and prognosis. The case of “TORCH”.
Evaluation of a child with a suspected birth defect is usually triggered by the presence of congenital anomalies, sought pre or post-birth, growth abnormalities (high or low stature, body segments disproportion or unexplained obesity), mental retardation or developmental delay, loss of vision, hearing loss, a family history of consanguinity or a known inheritable condition running in the family.
In infants with a birth defect, many times clinicians need to rule out a congenital infection. The diagnostic algorithm must include an exhaustive review of the maternal and perinatal history, but more laboratory diagnostic tools have become available. This has led to an increased sensitivity and specificity in the diagnosis of congenital infections and an increase in the observed prevalence of perinatal infections. But it has also uncovered a wider spectrum of these diseases (Boppana, 2017), what relates back to the 80% of birth defects with unknown aetiology
A “TORCH” diagnosis can be difficult, since many of the infectious and non-infectious clinical syndromes that present in the immediate neonatal period overlap in their semiology. Furthermore, sometimes there is no evidence of the infection until years later, when it is too late for an intervention. Hence, early recognition, including prenatal screening, is key. Fortunately for many of the pathogens associated to congenital infections, like Herpes simplex and Cytomegalovirus, treatment or prevention strategies and national and international standards and protocols are already available.
In the case of Toxoplasmosis, most clinicians would take a ‘risk-based’ approach and test the newborn based on ultrasound described findings (i.e., hydrocephalus and cerebral, hepatic or splenic calcifications). Maternal screening includes serology and molecular biology testing. PCR of the amniotic fluid obtained by amniocentesis at 18 weeks’ gestation can confirm foetal infection and guide medical therapy. Observational data suggest maternal therapy decreases foetal infection and the incidence of serious neurologic sequelae in the newborn (El Bissati, 2018).
There is no routine screening protocol for Parvovirus in pregnant women. Maternal symptoms, suspicious finding (i.e., Hydrops fetalis) on routine or screening ultrasound or a known maternal exposure might trigger testing for the infection. Monitoring for Hydrops fetalis and anaemia is recommended for at least 12 and up to 20 weeks after exposure (Crane, 2014). In utero foetal transfusion is the treatment of choice. It targets the anaemia and subsequent foetal hydrops, reducing foetal death.
In the case of HIV, testing algorithms and prevention protocols have had a significant impact on perinatal HIV thanks to the identification of maternal infection. Similarly, in the past women were screened for hepatitis B surface antigen (HBsAg) only if they fell into a high-risk group based on immunization status, history of exposure to blood products, intravenous drug use, etc. However, less than 60% of HBsAg carriers were identified using the screening criteria. Currently it is recommended that all pregnant women be screened for HBsAg at the first prenatal visit.
Teratogens and development of preventive or therapeutic strategies.
Once the teratogenic mechanism of action of an environmental factor is known, different means of prevention may be elucidated.
There are some very effective examples of teratogenesis prevention strategies in humans:
- Rubella immunization before childbearing age – it has practically eradicated the Congenital Rubella Syndrome (Bouthry, 2014).
- Supplementation of folic acid can prevent a large proportion of neural tube defects. The Prevention of Neural Tube Defects by Inositol (PONTI) pilot study should provide evidence to further evaluate inositol for primary prevention of NTD (Greene, 2016).
- Prevention of mental retardation by table salt supplementation with iodine in iodine-depleted regions (Syed, 2015).
- Glycaemic control during pregnancy.
- Avoiding alcohol drinking during pregnancy.
Prevention of teratogenesis in experimental animals include:
- Folinic acid pre-treatment of VPA-exposed mice. It reduced foetal resorptions and skeletal malformations. Pre-treatment with vitamins B6 and B12 protected mice against VPA – induced exencephaly and kidney malformations (Ornoy, 2018).
- Prevention of Autism Spectrum Disorders (ASD) like behaviour in mice postnatally injected with VPA, using piperine and green tea extracts (Ornoy, 2019b).
- Positive results in hyperglycaemic rodents models by feeding them arachidonic acid, myo-inositol and other nutritional agents (Dong, 2016).
- Foetal Alcohol Spectrum Disorder (FASD) prevention, using nutritional supplementation, VIP related peptides, antioxidants and folic acid (Zhang, 2018).
Conclusions
I believe the role of environmental factors on birth defects might be underestimated, considering the proportion of cases without a known aetiology. The study of teratogens still relies primarily on the use of in vivo animal-based assays, which are limited in their capacity to mimic human development.
As reviewed, recent work has focused on simulating organ development with organoid cultures. The use of organoids for teratogen evaluation is not widely extended yet, but advances in simulating the development of the brain, heart, kidneys, and eyes indicate a wider use may not be far.
Advances in human embryo culture and eventually the development of artificial embryos will provide models that can more accurately resemble early in-vivo development. The goal is to have a prototype to create an assay that reliable, high-throughput and consistent. However, human embryo models raise many ethical concerns.
I have tried to show that the study of certain environmental factors linked to birth defects has contributed to our current understanding of human embryonic development and the pathogenesis of congenital anomalies, while at the same time it has helped to improve the clinical diagnosis and prognosis of congenital diseases and the development of therapeutic strategies.
Nevertheless, research can reduce the global burden of birth defects even further by generating new preventive strategies against congenital infection, teratogens and other environmental factors, and potentially benefit millions worldwide. No disease is so rare as to deserve none of our attention.
References
- AKIMOVA, D., WLODARCZYK, B. J., LIN, Y., et al. (2017). Metabolite profiling of whole murine embryos reveals metabolic perturbations associated with maternal valproate-induced neural tube closure defects. Birth defects research, 109 (2), 106–119.
- AREF-ESHGHI E., SCHENKEL L.C., CARERE D.A., et al. (2018) Epigenomic Mechanisms of Human Developmental Disorders. In Epigenetics in Human Disease. Second Edition. 837-859.
- BOPPANA, S.B., BRITT, W.J., FOWLER, K., et al. Pathogenesis of Non-Zika Congenital Viral Infections, The Journal of Infectious Diseases 216 (10): S912–S918.
- BOURNE, H., DOUGLAS, T. and SAVULESCU, J. (2012) Procreative beneficence and in vitro gametogenesis. Monash Bioeth Rev 30: 29–48.
- BOUTHRY, E., PICONE, O., HAMDI, G., et al. (2014), Rubella and pregnancy: diagnosis, management and outcomes, Prenat Diagn, 34, 1246– 1253
- CABRERA, R.M., WLODARCZY, B.J. and FINNELL, R.H. Elucidation of FolateMediated Cascades in the Developing Neural Tube: Congenital Malformations Induced by Methyltransferase Inhibition. Journal of Molecular and Clinical Medicine, 2018, 1(2): 119-126.
- CHRISTIANSON, A., HOWSON, M. and MODELL, B. (2006) In March of dimes global report on birth defects. The hidden toll of dying and disabled children. White Plains, NY: March of Dimes Birth Defects Foundation.
- CUGOLA, F.R., FERNANDES I.R., RUSSO, F.B., et al. (2016) The Brazilian Zika virus strain causes birth defects in experimental. Nature 534 (7606): 267–271.
- CUI LI, DAN XU, QING YE, et al. (2016) Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell 19 (1): 120 -126.
- DI LULLO, E. and KRIEGSTEIN, A.R. (2017) The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. 18, 573–584.
- DAOYIN DONG, D., REECE, E.A., LIN, X. et al. (2016) New development of the yolk sac theory in diabetic embryopathy: molecular mechanism and link to structural birth defects. American Journal of Obstetrics and Gynecology 214 (2): 192-202.
- DUDGEON, J.A. (1967) Maternal rubella and its effect on the foetus. Arch Dis Child 42 (222):110–25.
- EL BISSATI, K., LEVIGNE, P., LYKINS, J., et al. (2018). Global initiative for congenital toxoplasmosis: an observational and international comparative clinical analysis. Emerging microbes & infections, 7(1), 165
- ESHRAGHI, A. A., LIU, G., KAY, S. S., et al. (2018). Epigenetics and Autism Spectrum Disorder: Is There a Correlation? Frontiers in cellular neuroscience, 12, 78.
- FATHE, K., PALACIOS, A. and FINNELL, R. H. (2014). Brief report novel mechanism for valproate-induced teratogenicity. Birth defects research. Part A, Clinical and molecular teratology, 100(8), 592–597.
- FRIEDMAN, C.E., NGUYEN, Q. LUKOWSKI, S.W. et al. (2017) Cardiac directed differentiation using small molecule Wnt modulation at single-cell resolution. BioRxiv 229294 (doi: https://doi.org/10.1101/229294).
- GÖTTLICHER, M., MINUCCI, S., ZHU, P. et al. (2001). Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. The EMBO journal, 20(24), 6969–6978.
- GREENE, N. D., LEUNG, K. Y., GAY, V., et al. (2016). Inositol for the prevention of neural tube defects: a pilot randomised controlled trial. The British Journal of Nutrition, 115(6), 974–983.
- HANSON, J. W., STREISSGUTH, A.P. and SMITH, D.W. (1978) The effects of moderate alcohol consumption during pregnancy on fetal growth and morphogenesis. Journal of Pediatrics 92: 457-60.
- JOHANNESSEN, S. I. AND LANDMARK, C. J. (2010). Antiepileptic drug interactions – principles and clinical implications. Current neuropharmacology, 8(3): 254–267.
- KELLY, T.F. (2018) In Avery’s Diseases of the Newborn. Tenth Edition.
- MILLS, R.J., TITMARSH, D.M., KOENIG, X., et al. (2017) Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci USA 114, pp. E8372-E8381
- MOREAU, J.L., KESTEVEN, S., MARTIN, E. M., et al. (2019) Development 146: 1 – 13.
- NIO, Y. and TAKEBE, T. (2019) Organoid Models of Development and Disease Towards Therapy. In: Inoue H., Nakamura Y. (Eds.) Medical Applications of iPS Cells. Current Human Cell Research and Applications. Springer, Singapore.
- ORNOY, A., WEINSTEIN‐FUDIM, L. and ERGAZ, Z. (2017), Antidepressants,
- Antipsychotics, and Mood Stabilizers in Pregnancy: What Do We Know and How Should We Treat Pregnant Women with Depression. Birth Defects Research, 109: 933-956
- ORNOY, A., KOREN, G. and YANAI, J. (2018) Is post exposure prevention of teratogenic damage possible: Studies on diabetes, valproic acid, alcohol and anti folates in pregnancy: Animal studies with reflection to human. Reproductive Toxicology 80: 92104.
- ORNOY, A., WEINSTEIN‐FUDIM, L., TFILIN, M., et al. (2019a) S-adenosyl methionine prevents ASD like behaviors triggered by early postnatal valproic acid exposure in very young mice. Neurotoxicology and Teratology 71: 64-74.
- ORNOY, A., WEINSTEIN‐FUDIM, L. and ERGAZ, Z. (2019b) Prevention or Amelioration of Autism-Like Symptoms in Animal Models: Will it Bring Us Closer to Treating Human ASD? Int J Mol Sci 20(5): 1074.
- RASMUSSEN, S.A., and FRIEDMAN, J.M. (2011). Emerging issues in teratology: an introduction. American journal of medical genetics. Part C, Seminars in medical genetics, 157 C (3), 147–149.
- SAVULESCU, J., PUGH, J., DOUGLAS, T. et al. (2015) Protein Cell 6: 476.
- SCHACHTMAN, N.A. (2016) Drug-Induced Birth Defects. Exploring the Intersection of Regulation, Medicine, Science, and Law. An Educational Module. Committee on Preparing the Next Generation of Policy Makers for Science-Based Decisions. Committee on Science, Technology, and Law.
- SCHLEISS, M.R. and MARSH, K.J. (2018) In Avery’s Diseases of the Newborn. Tenth Edition. 482-526.
- SCHULPEN, S. H.W., PENNINGS, J.L.A. and PIERSMA, A.H. (2015) Gene Expression Regulation and Pathway Analysis after Valproic Acid and Carbamazepine Exposure in a Human Embryonic Stem Cell-Based Neurodevelopmental Toxicity Assay, Toxicological Sciences 146 (2): 311–320.
- SHAO, Y., TANIGUCHI, K., GURDZIEL, K., et al. (2017) Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nature Materials 16: 419–425.
- SRINIVASAN, A. and TOH, Y. C. (2019) Human Pluripotent Stem Cell-Derived Neural Crest Cells for Tissue Regeneration and Disease Modeling. Frontiers in molecular neuroscience, 12, 39.
- SYED, S. (2015) Iodine and the “Near” Eradication of Cretinism. Pediatrics 135 (4) 594596.
- TIBONI, G.M. and PONZANO, A. (2015) Prevention of valproic acid-induced neural tube defects by sildenafil citrate. Reproductive Toxicology 56: 175-179.
- TOMSON, T.B. and PERUCCA, E. (2016) Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time‐honored drug. Lancet Neurol 15: 210–218.
- VOLMAR, C.H., and WAHLESTEDT, C. (2015) Histone deacetylases (HDACs) and brain function. Neuroepigenetics 1: 20-27.
- WHEELER, A.C. (2018) Development of infants with congenital Zika syndrome: what do we know and what can we expect? Pediatrics 141(Suppl 2): S154–60.
- XU, M., LEE, E.M., WEN, Z. et al. (2016) Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med 22 (10):1101–1107.
- ZHANG, Y., WANG, H., LI, Y. et al. (2018) A review of interventions against fetal alcohol spectrum disorder targeting oxidative stress, International Journal of Developmental Neuroscience 71: 140-145.
Cite This Work
To export a reference to this article please select a referencing style below:
Related Content
All TagsContent relating to: "research"
Nursing research can be defined as any scientific (i.e. systematic) enquiry into the effectiveness or value of nursing practice. It denotes any empirical evidence on which nursing care is based. This includes both quantitative and qualitative research evidence.
Related Articles


