Periodontal Disease and Its Implications on Human Health
From the Egyptians of Dayr alBarshā, Egypt, to modern humans, periodontal disease has affected human health in several observable and predictable ways. The link between periodontal disease and health is being investigated at both micro and macro levels by what authors He and Shi describe as “genetic mapping” and by three-dimensional biofilms models, a process used by Professor Georgios Belibasakis of Karolinska Institute at Huddinge, Sweden (2009; Sternudd, 2016). Connections between the two will not only provide valuable insight to periodontal disease, but also diseases of the heart, respiratory system, nervous system, and all other bodily systems. In my paper, I will briefly discuss prevalent risk factors of periodontal disease, explain processes by which the disease is transmitted, examine the signs and symptoms, discuss feasible and practical prevention strategies and treatment options, provide an outlook of the disease on a domestic scale, and establish a connection between periodontal disease and a patient with Alzheimer’s disease.
Risk Factors
Risk factors of disease determine an individual’s susceptibility to disease. Some factors can be modified, abolished, or interchanged, while other factors cannot. As a result, such factors classified into one of two categories: modifiable or non-modifiable.
Modifiable Risk Factors
As defined by authors Van Dyke and Dave (2005), modifiable risk factors are health risks caused by behavioral patterns – likely driven by some form of extrinsic motivation such as peer pressure, praise, or recognition – and/or environmental conditions relative to the patient (pg. 1). Such factors that increase one’s risk for periodontal disease include smoking, diabetes mellitus, the presence of microorganisms that “colonize the mouth”, and various psychological factors (stress or local stigma surrounding oral health) (Van Dyke & Dave). Modifiable risk factors are controllable and oftentimes dictated by one’s disposition. Risks of this type are complex, but with diligent research, medical professionals and researchers can confidently pinpoint specific risk factors that cause the disease.
Non-Modifiable Risk Factors
Conversely, non-modifiable risk factors are innate health risks within an individual not caused by extrinsic factors. Genetics, immune response of the host, age, sex, race or ethnicity, and socioeconomic status are among the most common non-modifiable risk factors for patients with periodontal disease (AlJehani, 2014). Despite the nature of genes and the immune response of the host, current research suggests developments in genetic engineering and improved methods of correction for immunocompromised individuals may not only improve the health of patients suffering from periodontal disease, but also patients suffering from heart disease, cystic fibrosis, Tay-Sachs disease, and Huntington’s disease.
Disease Transmission
According to the American Academy of Periodontology (n.d. – a), “periodontal disease is caused by inflammatory reaction to bacteria under the gums”. Transmission and influence of the disease is largely do genetic disorders and the transfer of saliva, oftentimes between family members and/or spouses.
Genetic Disorders
As previously mentioned, genetic mapping is an approach used to distinguish the link between health and disease. Essentially, genetic mapping does exactly what it says, it maps out genes. By using this approach, investigators significantly increase their ability to identify the specific genes involved in disease. In regards to periodontal disease, investigators have mapped out and isolated several gene clusters with the highest potential association to gum disease and have determined polygenic disorders as the most probable cause (Taba, Souza, Mariguela, 2012). As they studied these gene clusters, they found that the Interleukin-1 (IL-1) cluster plays a fundamental role in the development of periodontal disease. Through a delicate process of sorting and further identification, investigators discovered genes IL-1α and IL-1β directly aid in the development of the gum disease (Tarannum & Faizuddin, 2012). Such genes pass from parents to offspring relatively easy and are expressed in the majority of offspring whose parents carry the gene(s).
Transfer of Saliva
In addition to the inheritance of defective genes, the transfer of saliva is another route of transmission for the disease-causing microorganisms. According to Kim, J., Kim, C., and Camargo (2013), despite saliva’s antibacterial properties, it is able to carry specific biomarkers of periodontal disease (pg. 3). This simple finding demonstrates that the microorganisms that directly cause periodontal disease are present in saliva, even if it in minute amounts, and thus can be transmitted. Kissing, sharing personal items (toothbrush, dental tools), and sharing “eating utensils” are common ways saliva is transferred from person to person. Consequently, this is how periodontal disease spreads.
Signs and Symptoms
Commonly referred to as a “silent disease”, periodontal disease is generally unnoticeable throughout the early stages of it pathogenesis. Noticeable effects of the disease often appear late in late, more advanced stages of the disease and as a result, may cause significant health complications. Oral diseases with this distinctive characteristic are dangerous to individuals because they are virtually undetectable as they begin to spread through the mouth and body, infecting the connective tissues and mucous membranes of the mouth, as well as other tissues of the body, the subcutaneous fat of the cheeks, and alveolar bone of the teeth (The National Institute of Diabetes and Digestive and Kidney Diseases, 2014). Red, swollen gums, typically accompanied by oral pain and bleeding, is a telltale sign of an oral infection. If this is concurrent with other symptoms – receding gums, pus between teeth, persistent bad breath, and sores in mouth – periodontal disease has likely developed (American Academy of Periodontology, n.d. – b). Key symptoms such as these are indicative of periodontal disease and must be treated with urgency to prevent further damage to the mouth, gums, and teeth.
Prevention Strategies and Treatment
Prevention of periodontal disease refers to the precautions taken to inhibit the development of microorganisms and mineral buildup on the gums and teeth or its recurrence. In addition to basic oral hygiene, primary and secondary prevention strategies are often used to inhibit the spread of microorganism in the mouth, control plague (mineral) buildup, and control risk factors at an individual level. Furthermore, treatment of the disease integrates these levels of prevention into a complete system, which is then carried out by the individual and his or her dentist or periodontist.
Primary Prevention
According to Tonetti et al. (2015), primary prevention of periodontal disease refers to the “disruption of bacterial biofilm… [and inhibiting] the inflammatory process from destroying periodontal attachment” (pg. S6). Aside from daily cleaning, professional mechanical plague removal (PMPR) and local or systemic antibiotics are common primary prevention strategies that inhibit the development of periodontal disease.
Professional mechanical plague removal (PMPR). PMPR is a highly effective method to removal plague from teeth, making it one of the most common preventive strategies used by adults (Tonetti et al.). It incorporates a variety of hand and powered instruments intelligently designed to remove mineral deposits from the surface of teeth. PMPR is typically performed by a dentist through scaling or the polishing of teeth. In a study conducted by Zenthofer, Dieke, R., Dieke, A., Wege, Rammelsberg, & Hassel, (as cited in Tonetti et al., 2015), patients who underwent PMPR treatment experienced lower plague levels, less inflammation, and reduced bleeding (2013). Other studies on this strategy show similar outcomes, further reinforcing its capability to prevent periodontal disease.
Local and systemic antibiotics. Local and systemic antibiotics is another popular method to combat periodontal disease. Disinfecting agents such as chlorhexidine and locally-derived antibiotics “disinfect bacterial reservoirs” in the mouth and minimize biofilm growth. (Scottish Dental Clinical Effectiveness Programme, 2014). These antibiotics work by inhibiting enzyme activity and protein synthesize of harmful microorganisms and prevent the development of additional microorganisms (Kapoor, Malhotra, Grover, V., & Grover, D. 2012). Despite its advantages, local and systemic antibodies should only be used after or in addition to PMPR, never by itself, as research points to its ineffectiveness as a stand alone therapy.
Secondary Prevention
In comparison to primary prevention, secondary prevention refers to the measures taken to prevent the disease from reoccurring after successful treatment. If a dentist determines his/her patient would benefit from further prevention measures, they will likely suggest the patient undergo periodontal therapy based on the patient’s need, family history, and severity of the disease.
Periodontal therapy. According to Manresa, Sanz-Miralles, Twigg, & Bravo (2018), periodontal therapy “aims to reduce the inflammatory response [of the gums], primarily through eradication of bacterial deposits”, a process similar to PMPR. Many local clinics, such as Pillar Dental in Sioux Falls, SD, offer periodontal therapy to patients because the process eliminates periodontal infection and prevents further alveolar bone loss, significantly reducing the probability of disease reoccurrence (Pillar Dental, n.d.). When used in addition to PMPR, periodontal therapy not only improves oral health, but also the patient’s quality of life
Domestic Outlook
It has been over 300 years since the first description of periodontal disease. From that moment on, the prevalence periodontal disease has been extensively researched, documented, and predicted by epidemiologists worldwide. In regards to the current and predicted prevalence of periodontal disease in the United States, the U.S. Department of Health and Human Services (HHS) and its major operating component, the Center for Disease Control and Prevention (CDC), is tirelessly working to predict the disease’s prevalence over the next 10 to 15 years.
So far, they have documented prevalence rates in various populations across the country. In a 2007 statistics report published by the CDC, they outline the prevalence of the periodontal disease among adults 20 to 64 years of age, characterizing each by sex, race and ethnicity, poverty status, education, and smoking history, from 1988 to 1994 and 1999 to 2004. The results are shown in Table 1.
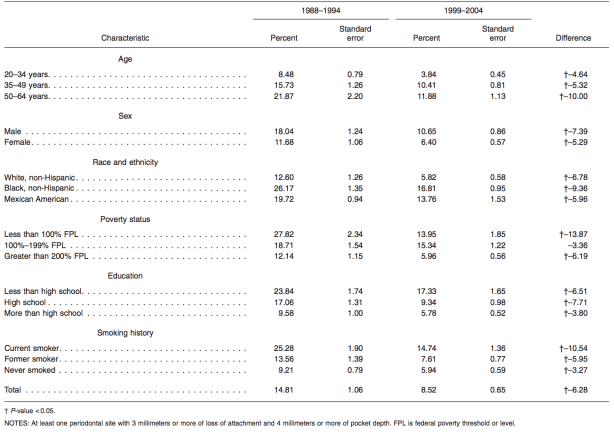
Analyzing the data, the prevalence of periodontal disease declines in all age groups from 1988 to 2004, with the largest decline documented in persons aged 50 to 64 years. Periodontal disease declined most in males, most in Black, non-Hispanics, most in persons whose poverty status is less than 100% of the federal poverty level (FPL), most in persons who graduated high school but did not pursue additional education, and most in current smokers. In total, the results showed an average decline of 6.28% across all populations sampled (CDC). If this trend continues, population health will continue to improve and periodontal disease will slowly be eradicated, whether that is through gene manipulation or by treatment and prevention measures.
Table 1. Prevalence of periodontal disease among dentate adults 20-64 years of age, by selected characteristics: United States, National Health and Nutrition Examination Survey, 1988-1994 and 1999-2004 (Center for Disease Control and Prevention, 2007).
Connection to Patient
The following is a hypothetical scenario intended for illustrative purposes only. It is unrelated to any patient as it may violate HIPAA.
In many studies, periodontal disease has been linked to a variety of comorbidities, for example cardiovascular disease, osteoporosis, bronchitis, Parkinson’s disease, and Alzheimer’s disease (Homstrup, Damgaard, Olsen, Klinge, Flyvbjerg, Nielsen, & Hansen, 2017). Various studies of periodontal disease and its association with other chronic disease reveal a high association between the two. In a study conducted by Cestari, Fabri, Kalil, Nitrini, Jacob-Filho, Tesseroli de Siqueira, et al. (as cited in Teixeiral, Saito, Matheus, Prediger, Yamada, Maia, & Lima, 2017), periodontal disease was shown to aid in the onset and progression of Alzheimer’s disease (AD) and vice versa (pg. 4). Patients oftentimes remember less of their day-to-day routine as AD develops and may forgot to accomplish simple tasks like brushing their teeth, flossing, etc. As a result, patients are at an increased risk of developing periodontal disease.
With this information, imagine an elderly patient, whom will be named Kate, with Alzheimer’s and periodontal disease. After months of pain, she finally decides to have her oral health examined by a local periodontist. During her visit, Kate’s periodontist probes Kate’s gums and discovers they bleed upon insertion of the probe. In addition, he also notices Kate’s gum are bright red and extremely sensitive to pressure. After the brief examination of Kate’s gums and teeth, Kate’s periodontist asks her about her oral hygiene routine. She vaguely describes her morning routine but cannot quite remember if she practices the same routine at night. She also has trouble recalling what toothpaste she regularly uses. Kate also mentions she smokes when she is stressed. Considering her symptoms, Kate’s periodontist believes she may have periodontal disease, largely caused by the deterioration of neurons in the pre-frontal cortex, a prominent effect of AD, which is causing her to neglect her daily oral hygiene routine. He also fears her use of tobacco is contributing to the disease’s aggressive behavior. Despite exhibiting key symptoms, additional testing is needed to accurately diagnose Kate. If it is determined Kate has periodontal disease, her periodontist will likely devise a personalized care plan that fits Kate’s needs, one that includes sessions of PMPR and a sufficient supply of local or systemic antibiotics. If treatment is successful, Kate can expect to participate in periodontal therapy until the potential for disease reoccurrence is low. With hard work, determination, and help from her periodontist, friends, and family, Kate can overcome a disease that affects the oral health millions of Americans across the United States.
Conclusion
To conclude, periodontal disease is an extremely dangerous disease capable of destroying the mouth, body, and in some instances, the mind. With consistent oral hygiene practices from individuals, primary and secondary treatment and prevention measures from dentists and periodontists, and further research from government agencies such as the CDC, the prevalence of periodontal disease will continue to decline, essentially leading to a healthier population that lives longer and experiences less diseases of the mouth, gums, and teeth.
References
- AlJehani, Y. A. (2014). Risk factors of periodontal disease: review of the literature. International Journal of Dentistry, 2014, 182513. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4055151/pdf/IJD2014-182513.pdf
- American Academy of Periodontology. (n.d. – a). Retrieved from https://www.perio.org/node/224
- American Academy of Periodontology. (n.d. – b). Retrieved from https://www.perio.org/consumer/gum-disease-symptoms.htm
- Center for Disease Control and Prevention. (2007). Prevalence of periodontal disease among dentate adults 20–64 years of age, by selected characteristics: United States, National Health and Nutrition Examination Survey, 1988–1994 and 1999–2004 [Digital image]. Retrieved from https://www.cdc.gov/nchs/data/series/sr_11/sr11_248.pdf
- He, X., & Shi, W. (2009). Oral microbiology: past, present and future. International Journal of Oral Science, 1(2), 47–58. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2949409/pdf/ijos20099a.pdf
- Homstrup, P., Damgaard, C., Olsen, I., Klinge, B., Flyvbjerg, A., Nielsen, C., & Hansen, P. (2017). Comorbidity of periodontal disease: two sides of the same coin? An introduction for the clinician. Journal of Oral Microbiology. 9(1). Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5508374/pdf/zjom-9-1332710.pdf
- Kapoor, A., Malhotra, R., Grover, V., & Grover, D. (2012). Systemic antibiotic therapy in periodontics. Dental Research Journal, 9(5), 505–515. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3612184/
- The National Institute of Diabetes and Digestive and Kidney Diseases. (2014). Diabetes, gum disease, & other dental problems. Retrieved from https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/gum-disease-dental-problems
- Pillar Dental (n.d.). Laser assisted periodontal therapy. Retrieved from http://pillardental.com/laser-assisted-periodontal-therapy.html
- Scottish Dental Clinical Effectiveness Programme. (2014). Prevention and treatment of periodontal disease in primary care – dental clinical guidance. Retrieved from http://www.sdcep.org.uk/wp-content/uploads/2015/01/SDCEP+Periodontal+Disease+Full+Guidance.pdf
- Sternudd, K., (2016). Studying oral disease in the laboratory with biofilm models. Retrieved from https://ki.se/en/research/studying-oral-disease-in-the-laboratory-with-biofilm-models
- Taba Jr, M., Souza, S., & Mariguela, V. (2012). Periodontal disease: a genetic perspective. Brazilian Oral Research, 26(1), 32-38. Retrieved from http://www.scielo.br/pdf/bor/v26nspe1/a06.pdf
- Tarannum, F., & Faizuddin, M. (2012). Effect of gene polymorphisms on periodontal diseases. Indian Journal of Human Genetics, 18(1), 9-19. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3385187/
- Teixeira, B., Saito, M., Matheus, F., Prediger, R., Yamada, E., Maia, C., & Lima, R. (2017). Periodontitis and Alzheimer’s Disease: A Possible Comorbidity between Oral Chronic Inflammatory Condition and Neuroinflammation. Frontiers in Aging Neuroscience, 9(327), 1-4. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5649154/pdf/fnagi-09-00327.pdf
- Tonetti, M., Eickholz, P., Loos, B., Papapanou, P., van der Velden, U., Armitage, G.,… Fundak, A. (2015). Principles in prevention of periodontal diseases – Consensus report of group 1 of the 11th European workshop on periodontology on effective prevention of periodontal and peri-implant diseases. Journal of Clinical Periodontology, 42(16), S5–S11. Retrieved from https://onlinelibrary.wiley.com/doi/pdf/10.1111/jcpe.12368
- Van Dyke, T., & Dave, S. (2005). Risk factors for periodontitis. Journal of the International Academy of Periodontology, 7(1), 3–7. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1351013/pdf/nihms4855.pdf
- Zenthofer, A., Dieke, R., Dieke, A., Wege, K., Rammelsberg, P. & Hassel, A. (2013) Improving oral hygiene in the long‐term care of the elderly ‐ a RCT. Community Dentistry and Oral Epidemiology, 41, 261–268. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23020631
Cite This Work
To export a reference to this article please select a referencing style below:

