Using Leishmania as an example, please describe the diagnostic pathway or algorithm, and innovations for diagnosis. Describe the major epidemiological changes in the last 20 years, and which of the diagnostic tests can also act as tools for surveillance.
In terms of the global burden of disease, the protozoan parasitic infection Leishmaniasis is regarded as the third most important arthropod-borne disease (Leitner et al., 2015). It is estimated that 12-15 million people suffer from Leishmaniasis infections (Torres-Guerrero et al., 2017), which can be divided into 4 forms; cutaneous, mucocutaneous, diffuse cutaneous and visceral. These infections are more prevalent in tropical and subtropical regions, with more than 2 thirds of new cases of Cutaneous Leishmaniasis (CL) in 2015 occurring in the same 6 countries (Norouzinezhad et al., 2016). Leishmaniasis is transmitted typically by a bite from an infected female fly to a mammalian reservoir species, primarily canine, and in endemic areas, humans are infected incidentally. (Steverding, 2017) Visceral leishmaniasis can be transmitted by intravenous drug use, transfusions and transplantations, and congenital infection (Meinecke et al., 1999). 30 species of phlebotomine sand flies are the vectors mainly responsible for the transmission of leishmaniasis in the new world regions, while in the old world the sand fly genera primarily responsible for the transmission of the disease is Lutzomyia. As these flies are weak fliers, they tend to live in close proximity to their breeding sites, meaning that incidence of the disease is closely related to the geographical distribution of the vector (Torres-Guerrero et al., 2017). In the past 20 years, one of the main changes in the epidemiology of Leishmania infections stems from the changing climate and its effect on the vector species. As the environment and thus habitats change and temperatures fluctuate this has had the potential to expand the geographical range of the fly species, leaving more individuals susceptible to infection (González, Paz and Ferro, 2014).
Not only does the incidence of the disease have a geographical correlation, but socioeconomic status can also influence the risk of disease. Areas of poverty in Asia, Africa and South America are usually most affected by the disease and prevalence in these countries is associated with malnutrition, migration and poor conditions of residency (Alvar, Yactayo and Bern, 2006). These areas usually have increased incidences of co-infection with autoimmune diseases such as HIV, and often lack resources in health care settings that do not allow for rapid diagnosis and treatment. Widespread, there is also no single therapy for the disease that allows satisfactory efficacy along with high levels of safety, meaning that efficient diagnosis is essential to prevent the rapid spread of the disease. Most human carriers of the disease are asymptomatic; this is especially common for Leishmania infantum in Southern Europe which labels the disease as a risk to public health (Yaghoobi-Ershadi et al., 2015). While most people infected with the disease will not display symptoms at first, Visceral Leishmania (VL) is an emerging condition affecting HIV positive patients in many Asian and African countries (Alvar et al., 2008). In the last 20 years, as transplantation medicine has been developed, many cases of VL have been reported in the treatment of immunosuppressed individuals (Bouchekoua et al., 2014). Diagnosis of the disease is paramount in these conditions for patients living or returning to areas that are endemic for Leishmaniasis, it is recommended that they undergo serological screening before immunosuppressive treatment or transplant surgery. There are 3 regarded ways in which suspected Leishmania cases can be diagnosed: parasitological, immunological and clinical. While the latter two methods are useful in combination to define clinical symptoms in endemic regions, smears using aspirations of bone marrow are used to identify the parasite in affected organ tissues and is essential to confirm a definitive diagnosis (Srivastava et al., 2011). However, these techniques are not without risk to the individual and cannot be performed practicably in a Low-Income country context as medical facilities are often lacking the funding and equipment required. WHO estimates that more than 350 million people across the globe are at risk from leishmaniasis (Bravo and Sanchez, 2003), and to control this threat there needs to be effective and practical forms of diagnosis available that can be used worldwide. These diagnostic treatments can not only improve individual health but can add to surveillance of the disease which is key in the epidemiology of parasitic neglected diseases such as leishmania, as it can lead to mapping of disease spread and contribute to innovations to control its transmission.
Diagnostic techniques to identify leishmania in human patients have changed substantially over the years; previously techniques where mainly invasive and relied heavily on the techniques of examining bone marrow and splenic aspirates (Zijlstra et al., 1992). Today, however, molecular techniques such as PCR and serological tests using recombinant kinesin antigen (rK39) are considered most effective despite risks relating to sensitivities and adaptability to the field. For diseases such as Leishmania, where endemicity plays a big role in the likelihood of symptoms being due to the disease, deciding on appropriate action based on clinical setting can be essential to the rapid diagnosis. Figure 1 shows a proposed algorithm for the diagnosis of Visceral Leishmania; by following an algorithm the most effective route of diagnosis for the disease can be identified.
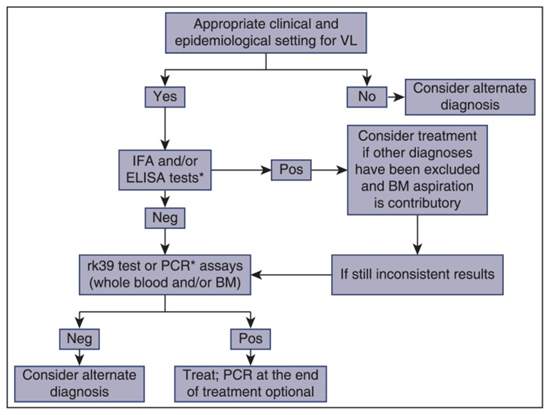
Figure 1: A proposed algorithm for the diagnosis of visceral leishmania (Georgiadou, Makaritsis and Dalekos, 2015)
An infection with VL induces an intense activation of innate immune pathways, alongside a stimulation of polyclonal B cells. This sometimes leads to the development of the hemophagocytic syndrome and production of autoantibodies which may event in misdiagnosis of the disease (Liberopoulos et al., 2013). This is particularly common in areas where the parasite has low endemicity, where medical professionals may diagnose the condition as autoimmune in favour of parasitism. Several serological tests that use specific antigens from the parasite are now available to increase efficiency and accuracy of diagnosis. Additionally, assays that use antigens from a whole parasite can be more sensitive but also display cross-reactivity with the antigens of malaria, Chagas disease and other endemic infections, meaning that misdiagnosis of the infection is common (Reed et al., 1990). Patients that clinically recover after successful VL treatment may also show positive serum antibodies against the parasite in the many months or years to follow (BERN et al., 2007), therefore assays such as this cannot be used accurately for diagnosis of treatment response. Nonetheless, many other serological diagnostic techniques can be effective for practical and rapid diagnosis. The direct agglutination test (DAT) is an effective tool that requires little equipment compared to enzyme-linked immunosorbent assays (ELISAs) and is practicable in low resources settings and contexts (Cañavate et al., 2011). Despite the wide ranges of these tools available, serological tests are not, by definition, suitable to prove the presence of active Visceral Leishmaniasis due to a wide range of non-specificity and cross-reactivity. Results of these tests must be taken into account in conjunction with epidemiological information on a case by case basis. Molecular diagnostic methods such as PCR, are usually highly specialised and can be sensitive for the detection of the DNA of the parasite, even down to particular species. They are therefore considered as essential in the diagnosis of a leishmania co-infection with the HIV virus. The sensitivity of PCR is even higher than the smears of cultures, but this can vary based on the type of tissue used. In 67 patients with active VL, it was shown that 100% of patients showed a sensitivity of PCR to both bone marrow and peripheral blood, suggesting that a combination of both specimens has the most success in diagnosing VL through PCR (Georgiadou and Dalekos personal unpublished data, 2014). It has been suggested that PCR assays may show efficacy in testing the response of a patient to leishmania therapy, however a lack of detailed standardization as well as a reduction of specificity are disadvantages of these tools in endemic areas as PCR cannot distinguish between asymptomatic infection and true active disease (Deborggraeve et al., 2008).
To carry out any of the above described tests, appropriate samples must be obtained from patients. When possible, spleen, bone marrow, liver and lymph nodes should be cultured in suitable growth media, where amastigotes in tissue are converted to motile promastigotes where they can then multiply in vivo (Sundar and Rai, 2002). While the sensitivity of these samples is marked, 70% and 96% for bone marrow and spleen aspirations respectfully (Zijlstra et al., 1992), the collection is not without risk. Splenic aspiration, in particular, is associated with perforation of organs such as the bowel or substantial haemorrhage (Thakur, 1997). The risks are reported mainly in Low-Income countries with inexperienced medical centres and complications can be fatal, therefore procedures are restricted to patients with no laboratory evidence of coagulopathy and should only be performed by an experienced medical practitioner. Although they are less sensitive, in clinical practice in nonendemic settings, bone marrow aspirates are preferred as they are much safer than splenic aspirates. Amastigotes are spherical or ovoid and are usually found in macrophages, characterised by a large nucleus and a rod-like organelle called the kinetoplast made up of DNA (Sunter and Gull, 2017). To give a definitive diagnosis of leishmania, visualisation of amastigotes under microscope imagery is required. The presence of the kinetoplast, and thus the amastigote, indicates the presence of Leishmania parasites and is key in the diagnosis of infection.
In recent history the epidemiology of Leishmania parasitism has changed significantly, leading to the need for the innovation of diagnostic methods to identify the disease in areas which have been nonendemic before. The incidence of Leishmaniasis can be influenced by changes in human settlement, most markedly urbanization of forested areas (Desjeux, 2001). Leishmaniasis is climate-sensitive due to a number of different factors in disease transmission and presentation of the clinical signs. Vectors of the fly and mammalian reservoir hosts are affected strongly by temperature, rainfall and humidity, altering population sizes and geographical distribution by influencing life cycles and habitat (Peterson and Shaw, 2003). Even small fluctuations in climate can have a pronounced effect on the lifecycle of the parasites themselves- leishmania promastigotes residing in the sand-fly vector can be altered so sufficiently that the parasite may be transmitted in areas that were not previously endemic for the disease. Additionally, changes in climate can be a social driver for the migration of people into new areas, especially in drought or famine conditions where compromised immunity due to poor nutrition may allow the parasite to infect individuals in areas that were once non-endemic. Currently, the geographical distribution of leishmania is wider than before, not only due to changes in climate but also due to the development of human behaviour and the way in which we use the environment. In Ethiopia, one-third of the country's landmass is suitable for the persistence of visceral leishmania caused by the L. donovani subspecies, with the mammalian reservoir hosts of dogs, bats and hyraxes being responsible for its maintenance in the population. Improvement in the type of vegetation through major agricultural schemes such as sugar cane farming has increased the density of vectors and the frequency of host-vector interactions. Additionally, the expansions of these projects have led to areas in which stray dogs are known to reproduce, as well as drawing more people to the area in search of work. (Gebremichael, 2018). Currently, there are 10 new sugar cane factories in Ethiopia and research has shown that the infection rate of CL and VL are often highest amongst people living at the edge of these development sites. In addition to this there this limited awareness of the disease amongst migrant workers and the local community, which is especially a problem as the disease is usually asymptomatic at first infection. These asymptomatic carriers in the population are unlikely to seek medical attention and so these cases are likely to go undetected until it is too late and either poses a risk to the individual or is spread further in the population. This means that diagnostic measures need to be in place, as well as education on the necessity of being tested for leishmania to prevent the disease from re-entering the individual’s country of origin on their return.
Surveillance of disease is one of the most important aspects of epidemiology, particularly in vector-borne diseases such as leishmaniasis that have many forms of the infection. Surveillance is typically undertaken in order to inform disease prevention methods, as well as providing data on the transmission that can act as a basis for control of the disease (Who.int, 2019). Surveillance can act as an early warning system to identify risks to public health, as well as guiding policy and strategies in the face of a disease outbreak by understanding the epidemiology of a condition. An effective surveillance system must investigate and confirm cases or outbreaks of disease, and create analysis reports to feedback data to an administrative level. As currently there are no preventative therapies or vaccines available to control the spread of the Leishmania, surveillance systems aim to identify changes in the trends of the disease to establish appropriate measures for its prevention. Surveillance by WHO is strengthened by a standardized approach to defining and managing cases by use of an online platform for data collection and dissemination at a global level, as well as regional and national (Fahrion et al., 2018). Case detection can take the form of passive detection, where patients take the initiative to visit health centres for diagnosis, or on active detection where a control programme identifies a community that may need screening for the disease. Passive detection most importantly relates to cases where the patient has a co-infection of Leishmania with HIV that is detected in a healthcare centre that is not run by a government organisation (Rabello, Orsini and Disch, 2003). Clinicians that identify such cases should notify the appropriate surveillance system; since epidemiologic surveillance is likely to be part of an existing system in the healthcare centre, passive detection is unlikely to require additional resources. Active detection mostly utilises serological screening in a camp-style environment over the winter months, taking into account the long incubation period of the Leishmania parasites that follows their transmission in warmer seasons (Piscopo and Mallia Azzopardi, 2007). In abiding by the concept of One Health principles (World Health Organization, 2019) the surveillance of leishmaniasis should include diagnosis of the canine hosts of the disease. Active detection of L. infantum infection in dogs has the greatest epidemiological value, as they represent a species that is suitable for identification of new areas of endemicity due to their status as a global reservoir host (Dantas-Torres, 2007). Additionally, in West Europe drugs for the treatment of Leishmaniasis in pet dogs are available and utilised to prevent the transmission of the disease from the infected animal to humans. In this context passive detection can also be used as people will frequently be encouraged to seek out veterinary care for their pets. Due to the variety of diagnostic tools available, the decision of whether to use passive or active surveillance is based heavily on resources made available by the country in question. Therefore, allowing the surveillance of the disease in the countries Low-income countries that tend to be most affected.
In regards to the surveillance of Leishmaniasis, it is clear that the last 20 years have challenged its control due to the changing epidemiological factors that have led to the more widespread prevalence of the disease. While aspects of epidemiology including co-infection, climate change and socio-economic change have expediated the influence of the disease in populations in previously non-endemic countries, innovations in diagnostic tools have been revolutionary in terms of monitoring the geographical distribution of the parasite, as well as noticing the condition in patients. Molecular and serological methods of diagnosis can contribute significantly to the timely diagnosis of leishmaniasis, attempting to decrease the marked morbidity and mortality of the disease. However, challenges still lie in the methods of sample collection that are not practicable in most endemic countries due to the lack of medical resources and additionally pose a risk to the individual. Although there is a great improvement in the field of parasite diagnosis, the persistence of the disease in mammalian reservoirs means that it will be impossible to eradicate globally without means of veterinary intervention. As the climate continues to change in the near future, innovations in diagnostic tools will need to take into account the predicted geographical spread of the disease as well as the ways in which transmission may be influenced. Since the dispersion of the infection in human populations is associated with distribution of vectors and host species, approaches aiming to prevent spread of the disease must take into account the stages in the parasites lifecycle that it is most transmissible in these reservoirs. Diagnostic tools that enable the surveillance of the disease can predict patterns in the spatial distribution of the parasite, allowing identification of the areas most at risk and potentially targeting measures of prevention and control with the hope of improving both animal and public health policy.
References
- Alvar, J., Aparicio, P., Aseffa, A., Den Boer, M., Canavate, C., Dedet, J., Gradoni, L., Ter Horst, R., Lopez-Velez, R. and Moreno, J. (2008). The Relationship between Leishmaniasis and AIDS: the Second 10 Years. Clinical Microbiology Reviews, [online] 21(2), pp.334-359. Available at: https://www.ncbi.nlm.nih.gov/pubmed/18400800.
- Alvar, J., Yactayo, S. and Bern, C. (2006). Leishmaniasis and poverty. Trends in Parasitology, [online] 22(12), pp.552-557. Available at: https://www.ncbi.nlm.nih.gov/pubmed/17023215.
- BERN, C., CHOWDHURY, R., BREIMAN, R., WAHED, M., WAGATSUMA, Y., SECOR, W., MAGUIRE, J., ALI, M., VAZ, L., AMANN, J., KURKJIAN, K., WILLIAMSON, J. and HAQUE, R. (2007). THE EPIDEMIOLOGY OF VISCERAL LEISHMANIASIS AND ASYMPTOMATIC LEISHMANIAL INFECTION IN A HIGHLY ENDEMIC BANGLADESHI VILLAGE. The American Journal of Tropical Medicine and Hygiene, [online] 76(5), pp.909-914. Available at: https://www.ncbi.nlm.nih.gov/pubmed/17488915/.
- Bouchekoua, M., Trabelsi, S., Ben Abdallah, T. and Khaled, S. (2014). Visceral leishmaniasis after kidney transplantation: Report of a new case and a review of the literature. Transplantation Reviews, [online] 28(1), pp.32-35. Available at: https://www.ncbi.nlm.nih.gov/pubmed/24321305.
- Bravo, F. and Sanchez, M. (2003). New and re-emerging cutaneous infectious diseases in Latin America and other geographic areas. Dermatologic Clinics, [online] 21(4), pp.655-668. Available at: https://www.ncbi.nlm.nih.gov/pubmed/14717406/.
- Cañavate, C., Bern, C., Chicharro, C., Blackstock, A., Alvar, J., Herrero, M., Aparicio, P., Cruz, I., Argaw, D., Nieto, J. and Mulugeta, A. (2011). Evaluation of Two rK39 Dipstick Tests, Direct Agglutination Test, and Indirect Fluorescent Antibody Test for Diagnosis of Visceral Leishmaniasis in a New Epidemic Site in Highland Ethiopia. The American Journal of Tropical Medicine and Hygiene, [online] 84(1), pp.102-106. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21212210/.
- Dantas-Torres, F. (2007). The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Veterinary Parasitology, [online] 149(3-4), pp.139-146. Available at: https://www.ncbi.nlm.nih.gov/pubmed/17703890.
- Deborggraeve, S., Boelaert, M., Rijal, S., De Doncker, S., Dujardin, J., Herdewijn, P. and Büscher, P. (2008). Diagnostic accuracy of a newLeishmaniaPCR for clinical visceral leishmaniasis in Nepal and its role in diagnosis of disease. Tropical Medicine & International Health, [online] 13(11), pp.1378-1383. Available at: https://www.ncbi.nlm.nih.gov/pubmed/18803611.
- Desjeux, P. (2001). The increase in risk factors for leishmaniasis worldwide. Transactions of the Royal Society of Tropical Medicine and Hygiene, [online] 95(3), pp.239-243. Available at: https://www.researchgate.net/publication/11852309_The_increase_in_risk_factors_for_leishmaniasis_worldwide.
- Fahrion, A., Gasimov, E., Joseph, S., Grout, L., Allan, M. and Postigo, J. (2018). Surveillance of leishmaniasis in the WHO European Region. Revue d'Épidémiologie et de Santé Publique, [online] 66, p.S394. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0398762018311295.
- Gebremichael, D. (2018). Zoonotic impact and epidemiological changes of leishmaniasis in Ethiopia. Open Veterinary Journal, [online] 8(4), p.432. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3602867/.
- Georgiadou, S., Makaritsis, K. and Dalekos, G. (2015). Leishmaniasis revisited: Current aspects on epidemiology, diagnosis and treatment. Journal of Translational Internal Medicine, [online] 3(2), pp.43-50. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4936444/.
- González, C., Paz, A. and Ferro, C. (2014). Predicted altitudinal shifts and reduced spatial distribution of Leishmania infantum vector species under climate change scenarios in Colombia. Acta Tropica, [online] 129, pp.83-90. Available at: https://www.sciencedirect.com/science/article/pii/S0001706X13002192.
- Leitner, W., Wali, T., Kincaid, R. and Costero-Saint Denis, A. (2015). Arthropod Vectors and Disease Transmission: Translational Aspects. PLOS Neglected Tropical Diseases, [online] 9(11), p.e0004107. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4652900/.
- Liberopoulos, E., Kei, A., Apostolou, F. and Elisaf, M. (2013). Autoimmune manifestations in patients with visceral leishmaniasis. Journal of Microbiology, Immunology and Infection, [online] 46(4), pp.302-305. Available at: https://www.ncbi.nlm.nih.gov/pubmed/22516744.
- Meinecke, C., Schottelius, J., Oskam, L. and Fleischer, B. (1999). Congenital Transmission of Visceral Leishmaniasis (Kala Azar) From an Asymptomatic Mother to Her Child. Pediatrics, [online] 104(5), pp.e65-e65. Available at: https://www.ncbi.nlm.nih.gov/pubmed/10545591.
- Norouzinezhad, F., Ghaffari, F., Norouzinejad, A., Kaveh, F. and Gouya, M. (2016). Cutaneous leishmaniasis in Iran: Results from an epidemiological study in urban and rural provinces. Asian Pacific Journal of Tropical Biomedicine, [online] 6(7), pp.614-619. Available at: https://www.sciencedirect.com/science/article/pii/S2221169116300880.
- Peterson, A. and Shaw, J. (2003). Lutzomyia vectors for cutaneous leishmaniasis in Southern Brazil: ecological niche models, predicted geographic distributions, and climate change effects. International Journal for Parasitology, [online] 33(9), pp.919-931. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0020751903000948.
- Piscopo, T. and Mallia Azzopardi, C. (2007). Leishmaniasis. Postgraduate Medical Journal, [online] 83(976), pp.649-657. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3202701/.
- Rabello, A., Orsini, M. and Disch, J. (2003). Leishmania/HIV co-infection in Brazil: an appraisal. Annals of Tropical Medicine & Parasitology, [online] 97(sup1), pp.17-28. Available at: https://www.who.int/leishmaniasis/burden/hiv_coinfection/en/ATMP2.pdf.
- Reed, S., Orge, M., Ghalib, H., Siddig, M., Badaro, R., Burns, J., Scott, J. and Shreffler, W. (1990). An Improved Serodiagnostic Procedure for Visceral Leishmaniasis. The American Journal of Tropical Medicine and Hygiene, [online] 43(6), pp.632-639. Available at: https://www.ncbi.nlm.nih.gov/pubmed/2267968.
- Srivastava, P., Dayama, A., Mehrotra, S. and Sundar, S. (2011). Diagnosis of visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene, [online] 105(1), pp.1-6. Available at: https://www.ncbi.nlm.nih.gov/pubmed/21074233/.
- Steverding, D. (2017). The history of leishmaniasis. Parasites & Vectors, [online] 10(1). Available at: https://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-017-2028-5.
- Sundar, S. and Rai, M. (2002). Laboratory Diagnosis of Visceral Leishmaniasis. Clinical and Vaccine Immunology, [online] 9(5), pp.951-958. Available at: https://www.ncbi.nlm.nih.gov/pubmed/12204943/.
- Sunter, J. and Gull, K. (2017). Shape, form, function and Leishmania pathogenicity: from textbook descriptions to biological understanding. Open Biology, [online] 7(9), p.170165. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5627057/.
- Thakur, C. (1997). A comparison of intercostal and abdominal routes of splenic aspiration and bone marrow aspiration in the diagnosis of visceral leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene, [online] 91(6), pp.668-670. Available at: https://www.ncbi.nlm.nih.gov/pubmed/9509175/.
- Torres-Guerrero, E., Quintanilla-Cedillo, M., Ruiz-Esmenjaud, J. and Arenas, R. (2017). Leishmaniasis: a review. F1000Research, [online] 6, p.750. Available at: https://www.ncbi.nlm.nih.gov/pubmed?Db=pubmed&Cmd=ShowDetailView&TermToSearch=28649370.
- Who.int. (2019). WHO | Public health surveillance. [online] Available at: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/en/ [Accessed 1 Dec. 2019].
- World Health Organization. (2019). One Health. [online] Available at: https://www.who.int/features/qa/one-health/en/ [Accessed 1 Dec. 2019].
- Yaghoobi-Ershadi, M., Marvi-Moghadam, N., Jafari, R., Akhavan, A., Solimani, H., Zahrai-Ramazani, A., Arandian, M. and Dehghan-Dehnavi, A. (2015). Some Epidemiological Aspects of Cutaneous Leishmaniasis in a New Focus, Central Iran. Dermatology Research and Practice, [online] 2015, pp.1-5. Available at: https://www.ncbi.nlm.nih.gov/pubmed?Db=pubmed&Cmd=ShowDetailView&TermToSearch=26483838.
- Zijlstra, E., Ali, M., El-Hassan, A., El-Toum, I., Satti, M., Ghalib, H. and Kager, P. (1992). Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Transactions of the Royal Society of Tropical Medicine and Hygiene, [online] 86(5), pp.505-507. Available at: https://www.ncbi.nlm.nih.gov/pubmed/1475815/.
Cite This Work
To export a reference to this article please select a referencing style below:

