Abstract
Lassa Fever (LF) is a haemorrhagic virus endemic in Western Africa. Symptoms can range from no symptoms to deafness, abortion and haemorrhage. Diagnosis can be difficult and treatment can only be effective if administered shortly after symptoms begin. The main route of transmission is zoonotic from Mastomys rodents but transmission between humans can occur. People who live in densely populated areas and improperly store food are at higher risk of attracting rats and coming into contact with urine/faeces. There are many control methods such as acquiring a cat/dog, using appropriate food storage and educating the local population. At this moment a vaccination is still unavailable however, there are 2 viable vaccines in the pipeline.
 Introduction
Introduction
Lassa virus is a single stranded RNA virus causing Lassa Fever (LF), an acute haemorrhagic illness[1]. It is part of the Arenaviridae family along with other viruses which cause haemorrhagic fever such as Machupa virus[2]. LF is endemic in West Africa and infects 100,000-300,000 people per year[3]. LF was responsible for 10-16% of all adult medical admissions and for ~30% of adult deaths in 1987 in Sierra Leonne2. 4/5 of people infected with Lassa virus will present no symptoms. LF has an incubation period between 6-212 days and, for the 20% who do experience symptoms, the early symptoms increase in intensity gradually starting from a fever, general malaise and other non-specific symptoms. In more severe cases symptoms can progress into facial swelling, vomiting, diarrhoea, fluid in the lung cavity, bleeding from the mouth, nose, vagina or gastrointestinal tract - this can then lead to a fall in blood pressure. As the disease progresses, symptoms can become even more serious including shock, seizures, coma and deafness[4]. These symptoms can be difficult to distinguish from other haemorrhagic fevers (such as Ebola) or other febrile diseases (Malaria). Haemorrhage appears in roughly 17% of cases2. In fatal cases, death usually occurs within 14 days of symptom presentation2. Around 5,000 deaths occur per year due to LF3. Studies suggest that an impaired or delayed cellular immunity causes fulminant viraemia and death.
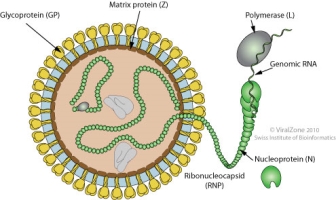
Figure 1- Structure of the Lassa virus. Single stranded RNA virus with transmembrane glycoproteins and matrix proteins.(Viralzone.expasy.org. (2019). Mammarenavirus ~ ViralZone page. [online] Available at: https://viralzone.expasy.org/83?outline=all_by_species [Accessed 19 Dec. 2019].)
Diagnosis and treatment
Diagnosis of LF can be difficult, and many patients are given antimalaria drugs and or antibiotics due to misdiagnosis. There are some clinical signs that are more specific to LF such as protein in urine, retrosternal pain, pharyngitis and fever2.
The four main techniques used to confirm diagnosis of LF are: Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR), antibody Enzyme-linked immunosorbent assay (ELISA), antigen detection tests and viral isolation by cell culture2.
Once the patient has been diagnosed, treatment must be given immediately. Supportive care with rehydration and symptomatic treatment has been shown to improve survival if it is given in the early stages. The most effective treatment is the antiviral drug ribavirin; studies show that it may reduce death by 90% if it is given to the patient within six days of the clinical presentation1. Use of ribavirin can lead to some side effects such as poor renal function.
Discussion
Epidemiology


2
Transmission
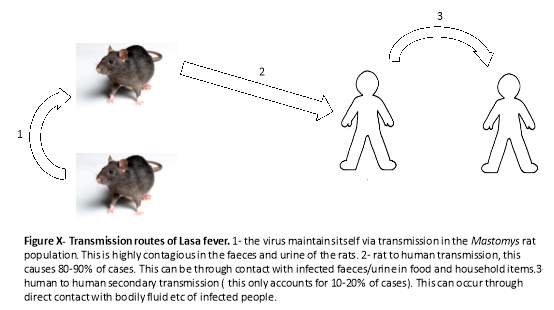
LF is a zoonotic disease which is endemic in Mastomy rat populations. The rats are widely distributed in Western and central Africa and are predominantly found around densely populated dwellings. The difficulty with this host is they do not become ill but shed the disease in urine and feaces2; they act as a reservoir host. Transmission between rats and humans can occur directly when the rats are eaten – they are thought of as a delicacy in Western Africa and are eaten by up to 90% of people in the main areas of infection[5]. Indirect contact is also an important route of transmission between humans and the Mastomy rats. Due to poor storage of food, rat faeces and urine are present in stored food such as grain. Due to inadequate housing the rats often are found living in houses in poorer communities and contact with infected urine/faeces with improper protection causes infection6.
Secondary transmission can occur between humans; this is usually spread by contact with bodily fluids of an infected person. LF can be spread indirectly due to the use of improperly sterilised medical equipment such as needles. Additionally, sexual transmission has been reported and there is evidence that the virus is excreted in semen for three months after infection1. Transmission often occurs in healthcare settings or when caring for a sick relative. Healthcare workers are especially at risk: 55% of healthcare workers encountered patients with LF4. In the recent outbreak in Nigeria (2016-2019) between January 1st and May 6th, 37 out of the 1,893 reported cases were in healthcare workers. This is usually due to improper precaution methods such as gloves and extra care when treating a patient with suspected/confirmed LF diagnosis4. However, on some occasions laboratory workers can become infected from improper handling of infected specimens2.
Figure 32
Endemic and epidemic patterns
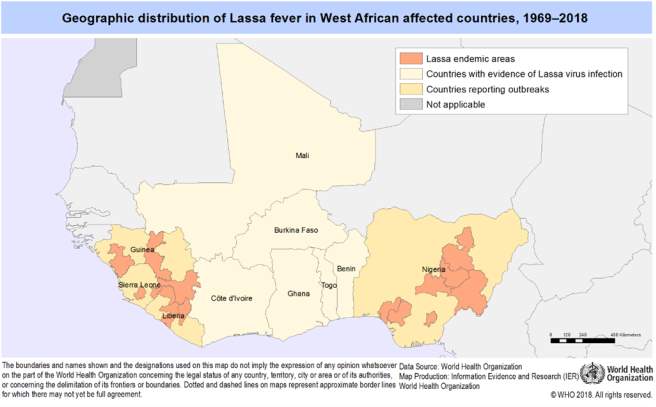
LF is known to be endemic in Nigeria, Togo, Benin, Ghana, Liberia, Sierra Leone, Guinea and Mali2. Countries where the virus is endemic tend to have a high prevalence of antibodies to the virus. Urban residential areas have a much higher prevalence of disease; around 73%[6] of cases were found to be in these areas as the more densely populated areas are a better feeding ground for the rats. There is more access to grain - this promotes more reproduction of the rats and greater transmission of disease. The World Health Organisation identifies the main risk groups as rural communities due to poor sanitation and or crowded living conditions.
LF seems to be prevalent in all age groups and both sexes4. Although most studies agree that age has no effect on LF, some papers have found different prevalence between the sexes. For example in Epidemiologic and Clinical Features of LF Outbreak in Nigeria, January 1–May 6, 2018 there appeared to be a higher prevalence in males than females, 62.1% to 37.9%4. Conversely, females were found to have higher prevalence in Lessons Learnt from Epidemiological Investigation of LF Outbreak in a Southwest State of Nigeria December 2015 to April 2016, 64% of infected people were found to be female[7]. This could be due to differences in exposure between genders or different reporting of cases between genders. Overall, most papers agree that there was no major difference in the incidence of disease between socio-demographic profiles7. LF is particularly dangerous in pregnant women, causing 25% of all maternal deaths in Sierra Leone. 87% of pregnant women who become infected with LF experience neonatal loss1.
Endemic and epidemic peaks generally occur seasonally; December to March/April is the dry season and is the annual peak of LF cases. This peak is caused by the higher risk of Mastomy rats entering houses in search of food, increasing the chance of humans coming into contact with their infected urine/faeces. Furthermore, there is some evidence that risk is increased in dry season, as the virus is able to survive outside the host for longer periods of time4 in the decreased humidity. However, the outbreak in Kenema (1999-2002) showed a peak between the wet and dry season. Evidence suggests that this was due to population movement resulting from civil unrest in the local area, causing overcrowding in refugees and poor sanitary conditions6.
Control
As the main route of transmission is zoonotic, it could be argued LF is easier to control than insect vector diseases. However, control relies on education and hygiene precautions which can be difficult to enforce in more rural areas or after of civil distress where refugees in crises live in close, poorly sanitised conditions. It can also be difficult as many people believe in herbal remedies or cannot afford treatment; this means that they will remain in the local community and spread the disease further1. Despite this, there are many prevention/control methods which have been proven very effective in minimising spread and transmission of LF. In the Nigerian outbreak in 2015-2016, there was a huge drive to educate the local population such as posters, TV and radio adverts8.
Promoting good hygiene practices in the community such as rodent proof containers for food (like grain), proper disposal of garbage far from living communities to decrease the attraction to rats, maintaining clean houses and the acquisition of cats to deter rats from entering the house. These practices greatly reduce the number of rats entering houses and spreading LF2. Although it is not plausible to eliminate the Mastomy rats from the wild, it is possible to minimise contact with the rats and their excrement/urine. It is also imperative that when rats are killed by a cat/human, they are disposed of properly. This may be difficult to enforce as rats are seen as a delicacy in many areas where LF is prolific. There is also some difficulty as rodent proof containers (such as plastic containers) are not widely available in the area, thus making it difficult to store food effectively[8]. There were some trials using anticoagulant rodenticide baits; these were effective while in use but, due to large litter sizes, these would need to be replaced regularly and are very expensive9. A more effective technique would be fixing cracks in house walls etc to improve housing and make it more difficult for rodents to enter. This is not only effective in the prevention of LF but other vector borne diseases such as the plague1.
In situations where healthcare workers/family members provide care for patients, there are many precautions to reduce the likelihood of secondary transmission. Avoiding contact with bodily fluid can be difficult so protective barriers such as gloves should be worn when caring for the patients and dealing with infected materials such as bedding, clothes and surface materials. The clinical guidelines state that healthcare workers must wear full sets of PPE (personal protective equipment) when “providing direct care to patients or managing medical or patient wastes, handling deceased bodies or cleaning”[9].
Many parts of Western Africa follow burial practices which encompass family members washing the body of the dead. This can cause further transmission of LF due to contact with bodily fluid from the deceased. Safe burial practices or use of PPE would reduce secondary transmission.
The use of control methods saw a significant drop in the recent outbreak in Nigeria, and with further education, there would be a further decrease in the number cases of LF per year.
Conclusion
Future
At present, there is no vaccine to prevent LF however, there is currently a large amount of research taking place into a potential vaccine using yellow fever as a vehicle1. A vaccination program would make a huge difference to many people in Western Africa yet, there are still some issues with the virus reactivating and causing disease. Vaccination programs can also be very expensive especially as 80% of the community would have to be vaccinated for eradication; it may be more cost effective to use control strategies. There is also another vaccine under development targeting the rodent vector[10]. This could potentially be difficult to distribute though. Although there is hope that a vaccine program will reduce LF deaths, it would be difficult to fully eradicate the virus due to the Mastomys rodent vector. There has also been heightened interest as viruses such as LF could be used as biological weapons, increasing the pressure for the development of a vaccine and better drug therapies1.
Bibliography
- Richmond, J. (2003). LF: epidemiology, clinical features, and social consequences. BMJ, [online] 327(7426), pp.1271-1275. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC286250/ [Accessed 18 Dec. 2019].
- Who.int. (2019). LF. [online] Available at: https://www.who.int/health-topics/lassa-fever/#tab=tab_1 [Accessed 18 Dec. 2019].
- Viprbrc.org. (2019). Virus Pathogen Database and Analysis Resource (ViPR) - Arenaviridae -. [online] Available at: https://www.viprbrc.org/brc/staticContent.spg?decorator=arena_lassa&type=About&subtype=LASSA [Accessed 18 Dec. 2019].
- Ilori, E., Furuse, Y., Ipadeola, O., Dan-Nwafor, C., Abubakar, A., Womi-Eteng, O., Ogbaini-Emovon, E., Okogbenin, S., Unigwe, U., Ogah, E., Ayodeji, O., Abejegah, C., Liasu, A., Musa, E., Woldetsadik,
- Dan-Nwafor, C., Furuse, Y., Ilori, E., Ipadeola, O., Akabike, K., Ahumibe, A., Ukponu, W., Bakare, L., Okwor, T., Joseph, G., Mba, N., Akano, A., Olayinka, A., Okoli, I., Okea, R., Makava, F., Ugbogulu, N., Oladele, S., Namara, G., Muwanguzi, E., Naidoo, D., Mutbam, S., Okudo, I., Woldetsadik, S., Lasuba, C. and Ihekweazu, C. (2019). Measures to control protracted large LF outbreak in Nigeria, 1 January to 28 April 2019. Eurosurveillance, [online] 24(20). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6530254/ [Accessed 19 Dec. 2019].
- Shehu, N., Gomerep, S., Isa, S., Iraoyah, K., Mafuka, J., Bitrus, N., Dachom, M., Ogwuche, J., Onukak, A., Onyedibe, K., Ogbaini-Emovon, E., Egah, D., Mateer, E. and Paessler, S. (2018). LF 2016 Outbreak in Plateau State, Nigeria—The Changing Epidemiology and Clinical Presentation. Frontiers in Public Health, 6(323).
- Isere, E., Fatiregun, A., Ilesanmi, O., Ijarotimi, I., Egube, B., Adejugbagbe, A. and Famokun, G. (2018). Lessons Learnt from Epidemiological Investigation of LF Outbreak in a Southwest State of Nigeria December 2015 to April 2016. PLoS Currents. [online] Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6063375/ [Accessed 16 Dec. 2019].
- Mari Saez, A., Cherif Haidara, M., Camara, A., Kourouma, F., Sage, M., Magassouba, N. and Fichet-Calvet, E. (2018). Rodent control to fight LF: Evaluation and lessons learned from a 4-year study in Upper Guinea. PLOS Neglected Tropical Diseases, [online] 12(11), p.e0006829. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6219765/ [Accessed 19 Dec. 2019].
- National guidelines for LF case management. (2019). [ebook] Jabi: LF technical working group. Available at: https://ncdc.gov.ng/themes/common/docs/protocols/92_1547068532.pdf [Accessed 19 Dec. 2019].
- Warner, B., Safronetz, D. and Stein, D. (2018). Current research for a vaccine against Lassa hemorrhagic fever virus. Drug Design, Development and Therapy, [online] Volume 12, pp.2519-2527. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6097522/ [Accessed 19 Dec. 2019].
[1] Richmond, J. (2003). LF: epidemiology, clinical features, and social consequences. BMJ, [online] 327(7426), pp.1271-1275. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC286250/ [Accessed 18 Dec. 2019].
[2] Who.int. (2019). LF. [online] Available at: https://www.who.int/health-topics/lassa-fever/#tab=tab_1 [Accessed 18 Dec. 2019].
[3] Viprbrc.org. (2019). Virus Pathogen Database and Analysis Resource (ViPR) - Arenaviridae -. [online] Available at: https://www.viprbrc.org/brc/staticContent.spg?decorator=arena_lassa&type=About&subtype=LASSA [Accessed 18 Dec. 2019].
[4] Ilori, E., Furuse, Y., Ipadeola, O., Dan-Nwafor, C., Abubakar, A., Womi-Eteng, O., Ogbaini-Emovon, E., Okogbenin, S., Unigwe, U., Ogah, E., Ayodeji, O., Abejegah, C., Liasu, A., Musa, E., Woldetsadik, S., Lasuba, C., Alemu, W. and Ihekweazu, C. (2019). Epidemiologic and Clinical Features of LF Outbreak in Nigeria, January 1–May 6, 2018. Emerging Infectious
Diseases, [online] 25(6), pp.1066-1074. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6537738/ [Accessed 17 Dec. 2019].
[5] Dan-Nwafor, C., Furuse, Y., Ilori, E., Ipadeola, O., Akabike, K., Ahumibe, A., Ukponu, W., Bakare, L., Okwor, T., Joseph, G., Mba, N., Akano, A., Olayinka, A., Okoli, I., Okea, R., Makava, F., Ugbogulu, N., Oladele, S., Namara, G., Muwanguzi, E., Naidoo, D., Mutbam, S., Okudo, I., Woldetsadik, S., Lasuba, C. and Ihekweazu, C. (2019). Measures to control protracted large LF outbreak in Nigeria, 1 January to 28 April 2019. Eurosurveillance, [online] 24(20). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6530254/ [Accessed 19 Dec. 2019].
[6] Shehu, N., Gomerep, S., Isa, S., Iraoyah, K., Mafuka, J., Bitrus, N., Dachom, M., Ogwuche, J., Onukak, A., Onyedibe, K., Ogbaini-Emovon, E., Egah, D., Mateer, E. and Paessler, S. (2018). LF 2016 Outbreak in Plateau State, Nigeria—The Changing Epidemiology and Clinical Presentation. Frontiers in Public Health, 6(323).
[7] Isere, E., Fatiregun, A., Ilesanmi, O., Ijarotimi, I., Egube, B., Adejugbagbe, A. and Famokun, G. (2018). Lessons Learnt from Epidemiological Investigation of LF Outbreak in a Southwest State of Nigeria December 2015 to April 2016. PLoS Currents. [online] Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6063375/ [Accessed 16 Dec. 2019].
[8] Mari Saez, A., Cherif Haidara, M., Camara, A., Kourouma, F., Sage, M., Magassouba, N. and Fichet-Calvet, E. (2018). Rodent control to fight LF: Evaluation and lessons learned from a 4-year study in Upper Guinea. PLOS Neglected Tropical Diseases, [online] 12(11), p.e0006829. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6219765/ [Accessed 19 Dec. 2019].
[9] National guidelines for LF case management. (2019). [ebook] Jabi: LF technical working group. Available at: https://ncdc.gov.ng/themes/common/docs/protocols/92_1547068532.pdf [Accessed 19 Dec. 2019].
[10] Warner, B., Safronetz, D. and Stein, D. (2018). Current research for a vaccine against Lassa hemorrhagic fever virus. Drug Design, Development and Therapy, [online] Volume 12, pp.2519-2527. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6097522/ [Accessed 19 Dec. 2019].
Cite This Work
To export a reference to this article please select a referencing style below:
Related Content
All TagsContent relating to: "epidemiology"
Epidemiology is the study and analysis of the distribution, patterns and determinants of health and disease conditions in defined populations. It is a cornerstone of public health, and shapes policy decisions and evidence-based practice by identifying risk factors for disease and targets for preventive healthcare
Related Articles


