Abstract
Cortical reorganisation offers a somewhat strong but ultimately incomplete explanation for phantom limb syndrome. Phantom limb syndrome describes the phenomena of experiencing sensations in a missing body part, usually following amputation. Previously, phantom limb syndrome has been explained by various mechanisms including psychological illness and irritation of neuromas. Wilder Penfield formed a map of motor and somatosensory localisation in the human brain. Tim Pons and his colleagues showed that following the loss of sensory input, the primary somatosensory cortex in macaque monkeys undergoes substantial reorganisation, with the sensory information from the monkey’s face invading the cortex of the paralysed hand. Ramachandran hypothesised that the Penfield map could also undergo similar reorganisation after amputation or deafferentation. This forms the basis of cortical reorganisation as an explanation for phantom limb syndrome and is supported by MEG and fMRI techniques. It also is strengthened by its applications to treatment (mirror therapy). However, work by Tamar et al. counters the idea of cortical reorganisation as a cause for phantom limb syndrome, weakening its explanatory power.
Is cortical reorganisation a strong explanation for phantom limb syndrome?
An individual with phantom limb syndrome experiences sensations in a missing part of the body. Currently, the main explanation for phantom limb syndrome is maladaptive cortical reorganisation in the cortex of the brain. It is suggested that once afferent input is lost from an amputation, cortical areas bordering the same amputation area will start to invade and gain control of the area, impacting the primary sensorimotor cortex. This is thought to result in phantom limb sensations. In the following essay, cortical reorganisation as an explanation for phantom limb syndrome will be evaluated.
Section 1
Phantom limb syndrome
Phantom limb syndrome describes the medical phenomena of experiencing painful or non-painful sensations in a body part that is not present. It is estimated that 60-80% of individuals with an amputation report experiencing phantom sensations in their amputated limb (Sherman, Sherman and Parker, 1984). Phantom sensations are most commonly felt after amputation of the limbs but have also been reported elsewhere, such as in the breast, eyes, teeth, bowel, bladder and penis. In a survey of phantom limb pain among American veterans, 78% reported phantom pain (ibid.). Phantoms are usually present initially for a few days or weeks but may last for years. For example, there are case studies of phantoms lasting 44 years (Livingston, 1945) and 57 years (Abbatucci, 1984). Commonly, phantom pain is described as throbbing, piercing, tingling or pins and needles sensations. Perhaps surprisingly, phantom limb pain has been reported in those with congenital limb absence as well as in amputees (Wilkins et al., 2004).
Earlier ideas on the explanation of phantom limb syndrome
Phantom limb sensation was once thought to be primarily a psychological illness, reflecting both the patient's grieving over the loss of the limb and his or her desire to believe that the limb was still present. A study did find the presence of depression to be a predictor of the severity of phantom limbs, however it was similarly associated with residual limb and back pain in amputees (Ephraim et al., 2005). With the accumulation of evidence from research over the past decades, the paradigm has shifted away from psychology and more towards changes at several levels of the neural axis, especially the cortex. Over recent years, peripheral and central neural explanations have gained a lot of support as proposed mechanisms.
Previously, irritation in neuromas (severed nerve endings) was believed to be the main explanation for phantom limbs. Following amputation, neuromas are terminated at the residual limb and may become inflamed. They were then thought to send abnormal signals to the brain which, due to being functionally nonsense, were interpreted by the brain as pain. However, although this idea appears logical, treatments based on this hypothesis tended to be unsuccessful. In extreme cases, in an attempt to remove the neuromas and provide temporary pain relief, surgeons would perform a second amputation. They found that the patients experienced increased phantom pain and the majority were left with the sensation of the original phantom limb, in addition to the new phantom stump, with a pain of its own. In a few cases, surgeons cut the sensory nerves leading into the spinal cord or even removed the part of the thalamus that receives sensory signals from the body (Ramachandran, 1998).
In the early 1990s, Ronald Melzack countered the idea that peripheral neuromas are the sole explanation of phantom limbs, instead emphasising that they are a part of a more complex picture. The occurrence of phantoms in those born missing limbs suggests that irritation in neuromas cannot be the main explanation for phantom limbs (Melzack, 1992). For example, there was a study of 13 congenital aplasics who experienced phantom limbs, seven of whom were able to move the phantom voluntarily, and four of whom had 'telescoped' phantoms (felt that the phantom limb was gradually shortening over time) (Weinstein, Sersen and Vetter, 1964).
Cortical reorganisation as an explanation for phantom limb syndrome
The homunculus
In a 1951 paper, Wilder Penfield mapped the cortical maps of the human brain and discovered that adjacent areas of the body would likely be adjacent on cortical maps. Penfield operated on patients under local anaesthetic and asked them what they felt following the use of an electrode to stimulate particular areas of the brain. Similar studies carried out previously had mainly relied on the use of animals, and hence their findings couldn’t necessarily be extrapolated to humans. Penfield collated data from 126 patients to develop the first comprehensive map of motor and somatosensory localisation in the human brain (Penfield, 1951). Known as the homunculus, this map showed the amount of cortical area dedicated to motor or somatosensory functions of each body part and is often depicted as a grotesquely distorted ‘little man,’ as seen in Figure 1.

Figure 1: The homunculus (Price-James, n.d.).
Cortical reorganisation in monkeys
In 1991, Tim Pons and his colleagues showed that following the loss of sensory input, the primary somatosensory cortex in macaque monkeys undergoes substantial reorganisation. They recorded signals from the brains of monkeys who had all the nerve fibres that carry sensory information from one arm into the spinal cord completely severed (dorsal rhizotomy). Eleven years later, when the researchers stroked the region corresponding to the affected hand, no activity was found. However, when they touched the monkey’s face, the cells corresponding to the ‘dead’ hand started vigorously firing (as well as cells corresponding to the face) (Pons et al., 1991). This showed that the sensory information from the monkey’s face had invaded the cortex of the paralysed hand, suggesting that you can alter the brain circuitry of an adult animal.
Section 2
Cortical reorganisation in humans
After reading about Pons’ research (see Section 1), V. S. Ramachandran hypothesised that the Penfield map (see Figure 2), shown in the human somatosensory cortex, could also undergo similar reorganisation after amputation or deafferentation (Ramachandran, 1998). This idea forms the basis of cortical reorganisation as an explanation for phantom limbs.
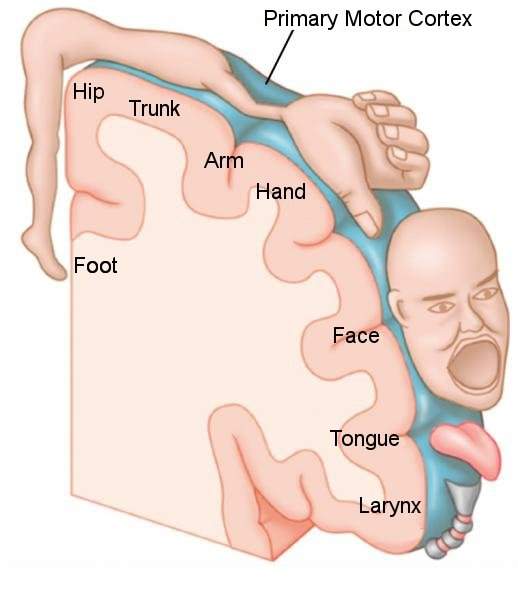
Figure 2: Penfield map (Carreiras, 2012)
Evidence for cortical reorganisation as an explanation for phantom limbs
Magnetoencephalogram (MEG) correlates of cortical reorganisation
Magnetoencephalography (MEG) is a non-invasive functional neuroimaging technique. By recording magnetic fields produced by electrical activity in the brain, one can map brain activity (Hämäläinen et al., 1993).
Recent improvements in MEG mean it is possible to form fine localisations of processing. This has allowed for the acquiring of extremely in depth somatosensory maps of body parts, including the hands and face (Gallen et al., 1993; Yang et al., 1993). These maps are so detailed that they are able to identify the cortical areas representing individual digits and even segments of digits. Using MEG studies, it was found that the remapping observed in Pons’ monkeys could also be seen in human amputees. In a study of four upper limb amputees, it was found that the sensory input from the face and upper arm had invaded the hand territory on the somatosensory map (Ramachandran, 1993b; Yang et al., 1994a, b). If one looks at Figure 2, this makes sense as the hand region is next to the arm and face regions on the Penfield map. Consequently, this provides evidence for large-scale topographical reorganisation in the adult human brain.
Behavioural consequences of cortical reorganisation
The MEG research formerly discussed implies that after reorganisation, the sensory input from the face will be sent to both the original cortical face area as well as the cortical area which used to receive information from the arm. This was tested in studies of patients after amputation.
Patient V.Q. was a smart and attentive 17-year-old who, 4 weeks prior to testing, had his left arm amputated 6 cm above the elbow. He had minor head injuries (including concussion) but at the time of testing he was mentally lucid, intelligent and fluent in conversation. He reported a telescoped phantom hand which felt as if it were attached just a few centimeters below his stump. To study localization of touch and light pressure, a cotton swab was brushed twice in rapid succession at various randomly selected points on the patient’s skin surface. During the entire procedure his eyes were closed and he was simply asked to describe any sensations that he felt and to indicate their perceived location. It was found that even stimuli applied to points far away from the amputation line were regularly systematically mislocalized to the phantom arm. Additionally, the distribution of these points was not random as they appeared to be grouped on the lower left side of the face (ipsilateral to amputation). There was a methodical one-to-one mapping between particular regions on the face and individual digits. Typically, the patient reported that he felt the cotton swab touching his face and, at the same time, experienced a tingling feeling in an individual digit. It was possible to plot 'receptive fields' for individual digits of the phantom left hand on his face surface, by repeatedly stroking the swab on his face . The margins of these fields were amazingly precise and stable over successive trials. Stimuli applied to other parts of the body (such as the shoulders, neck and tongue) were never mislocalized to the phantom hand. Roughly 7 cm above the amputation line, another group of points that elicited referred sensations was found. Like before there was a systematic one-to-one mapping. The thumb was portrayed medially on the anterior surface of the arm, and the little finger laterally, seemingly mimicking the phantom’s pronated position. Referred sensations in the index or ring fingers were evoked by the stimulation of points halfway between these two areas. An identical distribution of points was found when the whole procedure was repeated a week later. Hence, it suggests that these one-to-one correspondences are stable over time (Ramachandran et al., 1992a).
This study has high internal validity due to control of extraneous variables. For example, Patient V.Q. could not see during the procedure. This prevented his observations from impacting what he reported.
Notwithstanding this, it is important to note that this study only involves one participant, meaning that the findings are not necessarily generalisable, decreasing the population validity of the conclusions. Since Patient V.Q. suffered from minor head injuries, it is even harder to extrapolate the findings of his case to others because even if the injuries had no obvious effect on his behavior, the injuries may have confounded the results. Moreover, Patient V.Q. was only 17 and his age may have affected how his brain and perception changed following amputation.
Section 3
Strength: Supporting research
It has been shown using functional magnetic resonance imaging (fMRI) that significant links exist between different types of phantom limb pain and cortical reorganisation. fMRI identifies the variations in blood oxygenation and flow that are a consequence of neural activity in particular parts of the brain. Using 13 upper limb amputees, MacIver and his colleagues investigated activation during hand and lip movement, before and after intensive 6-week training in mental imagery. Before the training, motor and somatosensory cortices showed cortical reorganisation, displayed by the activation evoked during lip purse. The activation spread from lip area to hand area and correlated with pain scores. Following the training, patients declared a notable decline in intensity and unpleasantness of continual pain and aggravations, with a corresponding eradication of cortical reorganization. Prior to the clinical intervention, cortical reorganization witnessed in patients during various tasks decreased in relation to the decline in pain. However, there is no evidence that the link is causative (MacIver et al., 2008). The research has high internal validity due to standardisation and control of extraneous variables. For example, healthy volunteers were scanned twice to address the role of possible non-specific effects due to increased thresholds for neural activation because of, for example, familiarisation of the tasks. A strength of the research is that it relies on brain imaging techniques which are objective and less vulnerable to investigator bias than other techniques. Despite the small sample size decreasing external validity, the findings have high reliability since the conclusions are supported by other scientific research and clinical observations. Consequently, this research provides support of cortical reorganisation as an explanation for phantom limb syndrome, although it is impossible to identify a causative link.
Strength: Practical applications - Mirror therapy
In 1996, mirror therapy was developed by Ramachandran and Rogers-Ramachandran as a way to reduce phantom limb pain. A mirror is placed vertically on a table so that the reflection of the patient's intact limb is ‘laid’ over the perceived placement of the phantom (see Figure 3). The reflection of the undamaged body part helps reorganise and counter the incongruity between proprioception and visual feedback of the missing limb.

Figure 3: Demonstration of mirror box therapy (The Center for Brain and Cognition - UCSD, n.d.)
Foell and colleagues investigated the effects of daily mirror therapy over a four week period. The study used 13 patients who had chronic phantom limb pain after unilateral arm amputation. During an fMRI measurement, 11 participants carried out hand and lip movements before and after mirror therapy. The brain changes in relation to the mirror therapy were assessed using the location of neural activity within the primary somatosensory cortex. The mirror therapy led to a large reduction of phantom limb pain (average decrease of 27%). Significantly, fMRI data analyses showed a relationship between difference in pain after mirror therapy and a reversal of dysfunctional cortical reorganisation in the primary somatosensory cortex (Foell et al., 2013). Since the success of therapy is linked to cortical reorganisation, it implies that cortical reorganisation must play an important role in the development of phantom limbs.
This provides empirical support for cortical reorganisation as an explanation for phantom limb syndrome but also strengthens the theory by providing positive practical applications. The work of Foell et al. benefits from the objectivity of using brain imaging techniques, preventing bias from confounding the results. The generalisability of this study to other patients with phantom limb syndrome may be limited due to the small sample size. This research may only be helpful in understanding the mechanisms of phantom limb sensations in traumatic amputees, and ergo, may be irrelevant for those with congenital limb absence. Furthermore, some amputees experiencing phantoms do not get any relief from mirror therapy (Peterzell and Kennedy, 2016), limiting the usefulness of the treatment. It also suggests that the theory on which mirror therapy is based on (the idea of cortical reorganisation) must be incomplete or incorrect since mirror therapy does not always work.
Weakness: Opposing evidence
The maladaptive cortical reorganisation model predicts that representations of a missing hand should be reduced in the sensorimotor cortex of people suffering from more pain due to greater remapping.
In contrast, Tamar R. Makin and her co-investigators found, using fMRI scans, that amputees with ample phantom pain have stronger rather than weaker cortical representations of the missing body part, and no evidence of remapping. Moreover, while amputees and two-handed control subjects smacked their lips, there was, on average, no difference in cortical activation in the hand area between the two groups. This implies that cortical representation of the lips was not invading amputees’ missing-hand cortices. Activation during lip movement was not associated with pain intensity among the amputees.
Using two-handed control subjects, scans showed that when amputees moved their phantom hands, motor execution signals appeared within the cortex as if subjects were moving a real hand. This suggests that representation of the hand was maintained within the cortex in the amputees (Makin et al., 2013).
The work of Makin et al. had high levels of standardisation and control, increasing the internal validity of the study by eliminating extraneous variables. They used fMRI techniques, providing objectivity, yet, their work lacks reliability until there are replications in additional samples and studies.
Despite some flaws, Makin’s work still provides a valid argument against cortical reorganisation as an explanation for phantom limb syndrome.
Section 4
Conclusion
Cortical reorganisation is a somewhat strong explanation for phantom limb syndrome since it is well supported by a range of studies (e.g. MacIver et al., 2008, Ramachandran, 1993b and Yang et al., 1994a, b). However, it does not appear to be a complete explanation for phantom limbs. It seems that, alternatively, there are numerous mechanisms underlying phantom limb phenomena and it is very much unclear whether cortical reorganisation is a mechanism involved. Alternative proposals, such as the suggestions of Makin et al., definitely must be considered as they illuminate flaws in the cortical reorganisation explanation. Yet, both the cortical reorganisation explanation and the ideas of Makin et al. fall victim to bidirectional ambiguity. In regards to the former, one cannot tell whether cortical reorganisation leads to pain, or whether pain leads to cortical reorganisation. With Makin’s research, is the cortical representation of the missing hand maintained by pain signals, as Makin suggests, or is it maladaptive and driving the pain?
Understanding the mechanisms behind phantom limb syndrome is of extreme importance as it can help us to develop or improve treatments for the syndrome and aid our general scientific understanding of the brain’s structure and function. A survey found that 53% of patients with phantom limb pain and 38% with severe phantom limb pain had never been treated for the disorder (Hanley et al., 2006). Perhaps healthcare professionals are reluctant to address the issue due to the lack of understanding about what causes the phenomenon.
Bibliography
- Abbatucci, J. (1894). Etude psychologique sur les hallucinations des amputés. These de Bordeaux 1894, 33.
- Carreiras, C. (2012). Degree of representation of the different muscles of the body in the motor cortex.. [image].
- Ephraim, P., Wegener, S., MacKenzie, E., Dillingham, T. and Pezzin, L. (2005). Phantom Pain, Residual Limb Pain, and Back Pain in Amputees: Results of a National Survey. Archives of Physical Medicine and Rehabilitation, 86(10), pp.1910-1919.
- Foell, J., Bekrater-Bodmann, R., Diers, M. and Flor, H. (2013). Mirror therapy for phantom limb pain: Brain changes and the role of body representation. European Journal of Pain, 18(5), pp.729-739.
- Gallen, C., Sobel, D., Waltz, T., Aung, M., Copeland, B., Schwartz, B., Hirschkoff, E. and Bloom, F. (1993). Noninvasive Presurgical Neuromagnetic Mapping of Somatosensory Cortex. Neurosurgery, 33(2), pp.260-268.
- Hämäläinen, M., Hari, R., Ilmoniemi, R., Knuutila, J. and Lounasmaa, O. (1993).
- Hanley, M., Ehde, D., Campbell, K., Osborn, B. and Smith, D. (2006). Self-Reported Treatments Used for Lower-Limb Phantom Pain: Descriptive Findings. Archives of Physical Medicine and Rehabilitation, 87(2), pp.270-277.
- Livingston, K. (1945). The Phantom Limb Syndrome. A Discussion of the Role of Major Peripheral Nerve Neuromas. Journal of Neurosurgery, 2(3), pp.251-255.
- MacIver, K., Lloyd, D., Kelly, S., Roberts, N. and Nurmikko, T. (2008). Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain, 131(8), pp.2181-2191.
- Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Reviews of Modern Physics, 65(2), pp.413-497.
- Makin, T., Scholz, J., Filippini, N., Henderson Slater, D., Tracey, I. and Johansen-Berg, H. (2013). Phantom pain is associated with preserved structure and function in the former hand area. Nature Communications, 4(1).
- Melzack, R. (1992). Phantom limb pain. Patol Fiziol Eksp Ter., (4), pp.52-5.
- Paul, R., Goodman, H. and Merzenich, M. (1972). Alterations in mechanoreceptor input to Brodmann's areas 1 and 3 of the postcentral hand area of Macaca mulatta after nerve section and regeneration. Brain Research, 39(1), pp.1-19.
- Paul, R., Merzenich, M. and Goodman, H. (1972). Representation of slowly and rapidly adapting cutaneous mechanoreceptors of the hand in brodmann's areas 3 and 1 of Macaca Mulatta. Brain Research, 36(2), pp.229-249.
- Penfield, W. and Boldrey, E. (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain, 60(4), pp.389-443.
- Penfield, W. (1951). The Supplementary Motor Area of the Cerebral Cortex. A.M.A. Archives of Neurology & Psychiatry, 66(3), p.289.
- Peterzell, D. and Kennedy, J. (2016). Psychophysical investigations into Ramachandran’s mirror visual feedback for phantom limb pain: video-based variants for unilateral and bilateral amputees, and temporal dynamics of paresthesias. Electronic Imaging, 2016(16), pp.1-10.
- Pons, T., Garraghty, P., Ommaya, A., Kaas, J., Taub, E. and Mishkin, M. (1991). Massive cortical reorganization after sensory deafferentation in adult macaques. Science, 252(5014), pp.1857-1860.
- Price-James, S. (n.d.). [image].
- Ramachandran, V., Stewart, M. and Rogers-Ramachandran, D. (1992a). Perceptual correlates of massive cortical reorganization. NeuroReport, 3(7), pp.583-586.
- Ramachandran, V. (1998). The perception of phantom limbs. The D. O. Hebb lecture. Brain, 121(9), pp.1603-1630.
- Sherman, R., Sherman, C. and Parker, L. (1984). Chronic phantom and stump pain among american veterans: results of a survey. Pain, 18(1), pp.83-95.
- The Center for Brain and Cognition - UCSD (n.d.). Demonstration of mirror box therapy for phantom limb pain developed by Dr. V.S. Ramachandran at UCSD.. [image].
- Weinstein, S., Sersen, E. and Vetter, R. (1964). Phantoms and Somatic Sensation in Cases of Congenital Aplasia. Cortex, 1(3), pp.276-290.
- Wilkins, K., McGrath, P., Finley, G. and Katz, J. (2004). Prospective Diary Study of Nonpainful and Painful Phantom Sensations in a Preselected Sample of Child and Adolescent Amputees Reporting Phantom Limbs. The Clinical Journal of Pain, 20(5), pp.293-301.
- Yang, T., Gallen, C., Schwartz, B. and Bloom, F. (1993). Noninvasive somatosensory homunculus mapping in humans by using a large-array biomagnetometer. Proceedings of the National Academy of Sciences, 90(7), pp.3098-3102.
- Yang, T., Gallen, C., Schwartz, B., Bloom, F., Ramachandran, V. and Cobb, S. (1994a). Sensory maps in the human brain. Nature, 368(6472), pp.592-593.
- Yang, T., Gallen, C., Ramachandran, V., Cobb, S., Schwartz, B. and Bloom, F. (1994b). Noninvasive detection of cerebral plasticity in adult human somatosensory cortex. NeuroReport, 5(6), pp.701-704.
Works consulted
- Bailey, A. and Moersch, F. (1941). Phantom Limb. Canadian Medical Association, 45(1), pp.37-42.
- de Vignemont, F., Ehrsson, H. H., & Haggard, P. (2005). Bodily illusions modulate tactile perception.
- Current Biology, 15(14), pp.1286-1290.
- Farne, A., Roy, A. C., Giraux, P., Dubernard, J. M., & Sirigu, A. (2002). Face or hand, not both:
- Perceptual correlates of reafferentation in a former Amputee. Current Biology, 12(15), pp.1342-
- 1346.
- Flor, H., Elbert, T., Mühlnickel, W., Pantev, C., Wienbruch, C. and Taub, E. (1998). Cortical reorganization and phantom phenomena in congenital and traumatic upper-extremity amputees. Experimental Brain Research, 119(2), pp.205-212.
- Flor, H., Nikolajsen, L. and Staehelin Jensen, T. (2006). Phantom limb pain: a case of maladaptive CNS plasticity?. Nature Reviews Neuroscience, 7(11), pp.873-881.
- Funk, M., Shiffrar, M., & Brugger, P. (2005). Hand movement observation by individuals born without hands: phantom limb experience constrains visual limb perception. Experimental Brain Research, 164(3), pp.341-346.
- Haigh, R. C., McCabe, C. S., Halligan, P. W., & Blake, D. R. (2003). Joint stiffness in a phantom limb: evidence of central nervous system involvement in rheumatoid arthritis. Rheumatology (Oxford), 42(7), pp.888-92 . (3 cases where joint stiffness was perceived in both a normal and a phantom limb − and is therefore due to “secondary plastic changes in the CNS”)
- Krane, E. and Heller, L. (1995). The prevalence of phantom sensation and pain in pediatric amputees. Journal of Pain and Symptom Management, 10(1), pp.21-29.
- Ramachandran, V. S. (2005). Plasticity and functional recovery in neurology. Clinical Medicine, 5(4), pp.368-373.
- Roux, F. E., Lotterie, J. A., Cassol, E., Lazorthes, Y., Sol, J. C., & Berry, I. (2003). Cortical areas involved in virtual movement of phantom limbs: Comparison with normal subjects. Neurosurgery, 53(6), pp.1342-1352.
- Schicke, T., & Roder, B. (2006). Spatial remapping of touch: Confusion of perceived stimulus order across hand and foot. Proceedings of the National Academy of Sciences of the United States of America, 103(31), pp.11808-11813.
- Subedi, B. and Grossberg, G. (2011). Phantom Limb Pain: Mechanisms and Treatment Approaches. Pain Research and Treatment, 2011, pp.1-8.
- Topper, R., Foltys, H., Meister, I. G., Sparing, R., & Boroojerdi, B. (2003). Repetitive transcranial magnetic stimulation of the parietal cortex transiently ameliorates phantom limb pain-like syndrome. Clinical Neurophysiology, 114(8), pp.1521-30. (The results “support the concept that phantom pain is due to a dysfunctional activity in the parietal cortex.”
Cite This Work
To export a reference to this article please select a referencing style below:

