Abstract
Although measles is a vaccine-preventable disease with highly effective vaccination, it still remains a cause of a global outbreak and mortality. Measles caused around 109,638 deaths in 2017, and measles outbreaks have re-emerged in the United States and Venezuela due to population movement and increasing anti-vaxxers in recent years, implying the importation of endemics to other border countries. This essay aims to demonstrate the significant challenges, which can be difficulties to prevent the measles outbreak and mortality completely. To answer this question, this essay reviews the literature on epidemiology and control measures, classification and structure of measles, viral life cycle and pathogenesis, symptoms and immune responses, and diagnosis of measles. Hence, this essay found epidemiological challenges arising from vaccination programmes with measles-containing vaccine (MCV) coverage and biological and clinical barriers that contribute to severe pathogenesis of measles leading a cause of high mortality.
The results indicate that measles is a highly contagious disease due to the high basic reproductive number (R0) estimating 12 to 18, caused by the measles virus (MV) through airborne via respiratory tracts. MV has high stability in the airspace surviving for at least one hour. It has two significant proteins, such as haemagglutinin (H) protein and fusion (F) protein, which have an impact on severe pathogenesis, combining with CD 150 and nectin-4 in the host.
After infection of MV, the incubation period shows longer than the latent period, symptoms of measles include fever, conjunctivitis, Kopik’s spot, and rash. MV causes immune suppression such as lymphopenia with decreasing CD4 and CD8 T cells and B cells that result in opportunistic infections including otitis media, pneumonia, and diarrhoea, which can be the cause of increased mortality in childhood. Both detection of MV-RNA by reverse transcription PCR (RT-PCR) and detection of anti-MV IgM antibodies by enzyme immunoassay (EIA) are commonly used in the diagnosis of measles based on the high sensitivity and specificity. However, EIA and RT-PCR should be carried out with the adequate quality of samples collected timely and EIA can lead to false-negative and positive errors.
After the introduction of the highly effective vaccination of measles, the incidence and mortality rates of measles has been decreased considerably, and above 21 million deaths were prevented through the vaccination from 2000 to 2017. Thus, WHO recommends the vaccination to prevent measles through the implementation of Global Measles and Rubella Strategic Plan with MCV coverage. Although it had a tremendous achievement with the decreased incidence and mortality rates by 83% and 80% respectively between 2000 and 2017, WHO goal was not successful in 2015 owing to the static MCV1 (first dose) coverage over around one year and lower MCV2 (second dose) coverage (67%) in some countries including Mongolia.
On this basis, it is recommended that not only control measures including MCV coverage should be re-established in consideration of the cause of the recent outbreaks such as population movement and anti-vaxxers but also biological and clinical barriers should be taken into account with adequate researches for global measles eradication.
Introduction
Measles virus (MV) is a highly contagious infectious disease and can lead to death, especially among young children or in areas with low vaccination. According to Dabbagh et al. (2018), measles caused around 109,638 deaths in 2017, and approximately 1 in every 100 children will die from measles or its complications in low-income countries. Accordingly, various control measures including vaccination programmes, vitamin A therapy for young children, post-exposure prophylaxis for pregnant women and immunocompromised persons, and surveillances have been conducted for treatment and global measles eradication (Rota et al., 2016; Dabbagh et al. 2018; Strebel and Orenstein, 2019). Especially, among those control measures, vaccination programmes with measles-containing vaccine (MCV) coverage are regarded as the key control measure due to the fact that MV is a vaccine-preventable disease and more than 21 million deaths were prevented globally between 2000 and 2017 with a highly effective vaccination (Dabbagh et al., 2018).
However, recently, incidences of measles have increased in the United States and other countries, which contribute to an upward global trend in incidence from 2018 to 2019 (Strebel and Orenstein, 2019). Thus, it can be said that MV still remains a leading cause of epidemics with some limitations in global eradication. In this respect, this essay will summarise epidemiology and vaccination programmes of MV and demonstrate the challenge arising from MCV coverage in recent years, followed by classification and structure, viral life cycle and pathogenesis, symptoms and immune responses and diagnosis of measles with discussion of biological and clinical barriers which can be difficulties in global measles eradication.
Epidemiology and vaccination programmes
Madagascar, Ukraine, India, Brazil, Philippines, Venezuela, Thailand, Kazakhstan, Nigeria, and Pakistan were the countries reporting the largest number of measles cases in 2019 (WHO, 2019a). Although the major reason for measles epidemics is that those countries have weak health systems, WHO (2019b) argued that vaccine hesitancy due to the inadequacy of the information about vaccination is one of the top 10 global health threats in 2019. McLean et al., (2013) found that measles vaccine has considerable effectiveness when infants aged 12 months get vaccinated the first dose (median effectiveness, 93%) and the second dose in the next few years (median, 97%) with lifelong immunity (Strebel and Orenstein, 2019). Thus, WHO (2017) recommends two doses of vaccine as a standard care to prevent measles and reach herd immunity thresholds in leading to termination of the transmission. Based on these advantages of measles vaccination, WHO has implemented the Global Measles and Rubella Strategic Plan 2012-2020 with MCV coverage, which aims to achieve global elimination of measles in at least 5 WHO regions by 2020, and elimination is defined as the absence of measles endemic transmission for greater than or equal to 12 months in a geographic area (WHO, 2012). Hence, measles incidence and mortality decreased by 83% and 80% respectively between 2000 and 2017, attributed to increased MCV coverage through the WHO global plan (Strebel and Orenstein, 2019).
However, WHO goal was not successful in 2015 as a result of the lower MCV2 (second dose) coverage (67%) and the stagnated MCV1 (first dose) coverage over approximately one year (Dabbagh et al., 2018). For instance, in Venezuela, the measles outbreak has reoccurred since 2018 as measles transmission sustained for above 12 months, indicating that the endemic has led to outbreaks in the countries bordering Venezuela (ibid). In addition, MCV coverage alone is not always adequate to predict immunity. Especially, despite high MCV2 coverage rate (97%) in Mongolia, the average annual incidence rate was the highest accounting for 1732.6 per million cases among 123 countries from 2009 to 2018 (Javelle et al., 2019). Moreover, although these countries including Albania, Bangladesh, Belarus, Bhutan, Israel, Kazakhstan, Kyrgyzstan, Malaysia, North Macedonia, Portugal, Saudi Arabia, Slovakia, Tajikistan, Thailand, the Russian Federation, and the United Arab Emirates have MCV2 coverage above 95%, they showed similar or even higher incidence rates compared to other countries with MCV2 coverage below 50% from 2017 to 2019 (Figure 1).
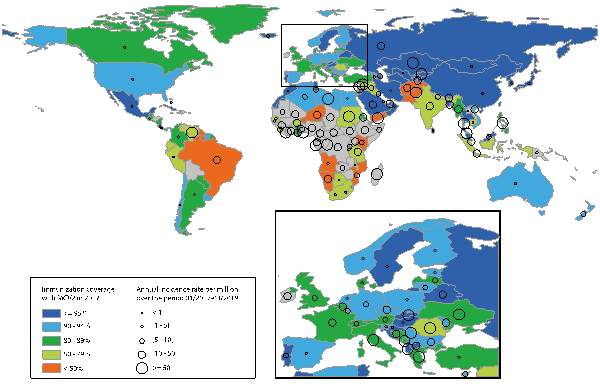
Figure 1. MCV2 coverage in 2017 and the average annual measles incidence rate per million from Jan 2017 to Mar 2019 (Javelle et al., 2019, p. 900).
These countries such as Albania, Bangladesh, Belarus, Bhutan, Israel, Kazakhstan, Kyrgyzstan, Malaysia, North Macedonia, Portugal, Saudi Arabia, Slovakia, Tajikistan, Thailand, the Russian Federation, and the United Arab Emirates have MCV2 coverage above 95% but higher incidence rates compared to other countries who have MCV coverage below 50% between 2017 and 2019.
Hence, MCV coverage can be no longer strongly associated with the elimination of measles outbreak causing of limitations. Although MCV coverage is necessary to reach herd immunity threshold leading to prevention of measles endemics owing to the high effectiveness of vaccination, when considering recent situation which is the contradiction between improving immunity and increasing incidence rates in some countries, it can be challenged whether continuing to increase MCV coverage is still the most effective scheme to eradicate measles. The outcomes of ineffective MCV coverage and the recent outbreaks in certain countries can be attributed to population movement including travelling and migration (Heywood, 2018; Javelle et al., 2019; Orsoo et al., 2019) and the growing trend of anti-vaxxers (Phadke et al., 2016).
Classification and structure of measles
MV is paramyxoviridae family and morbillivirus genus, and it has a non-segmented, negative-sense and single-stranded RNA genome of around 16,000 nucleotides surrounded by an outer envelope (Rota et al., 2016). The envelope has two types of proteins such as haemagglutinin (H) protein and fusion (F) protein. In fact, MV has serval different types of proteins (Figure 2) but H and F proteins are significant concerning pathogenesis as they have a function of attachment with and enter into host cells (Tahara et al., 2016). Particularly, H protein is related to immunisation or vaccination because the main antibody response is to H protein epitope in surface glycoproteins (Hashiguchi, Maenaka and Yanagi, 2011).

Figure 2. Measles virus structure and viral life cycle (Rota et al., 2016, p. 5).
Measles has four internal proteins, including matrix (M) protein, large (L) protein, phosphoprotein (P) and nucleocapsid (N) protein, and two external proteins, including fusion (F) protein and haemagglutinin (H) protein surrounding by lipid bilayer. F protein combines with signalling lymphocyte activation molecule (SLAM) on immune cells and H protein fuses with a membrane to enter into the host cell. In the cytoplasm, virus replication, transcription, and translation initiate in regular sequence. After interacting with new viral proteins and other eliminants, a newly made virus releases out.
Viral life cycle and pathogenesis
In terms of viral life cycle (Figure 2), when the virus comes into the host, the virus uses H protein to bind to CD 150 (i.e., SLAM; signalling lymphocyte activation molecule) on immune cells such as B and T cells or macrophages and dendritic cells (DCs), or nectin-4 (i.e., cellular adhesion molecule) on epithelial cell from the host cell. Once H protein on the virus binds with one target receptor on the host cell, F protein helps the virus fuse with the membrane, and the virus can get inside the host cell. Henceforth, virus replication and transcription of the viral genome initiate in the cytoplasm of the host cell, ultimately, a newly produced virus sends out of the cell and MV spreads through local tissues within days (Rota et al., 2016).
Pathogenesis is divided into 3 steps (Figure 3). The first step is entry. After MV is aspirated into the respiratory tract as MV is an airborne pathogen, the virus infects alveolar macrophages or DCs in conjunctiva, upper and lower respiratory tracts using SLAM as a receptor (Laksono et al., 2016). The second step is dissemination. Infected macrophages or DCs move to the subepithelial cell layer and transmit the virus to epithelial cells in different organs or tissues such as primary, secondary and tertiary lymphoid tissues using nectin-4 as a receptor. As these lymphoid tissues have abundant SLAM, they are the major sites to amplify the virus (ibid). With the cell-to-cell virus dissemination, clinical measles begins with the appearance of Koplik’s spots and terminates with the emergence of the maculopapular skin rash after a few days (Griffin, 2013). Occasionally, MV infects to central nervous system (CNS), which is a serious threat to the patients but still unclear how MV can access to CNS (Laksono et al., 2016). The last step is transmission. After MV is multiplied in organs or tissues, a large amount of progeny viruses is released into the respiratory tract, which induces coughs spreading MV particle into the air (ibid).
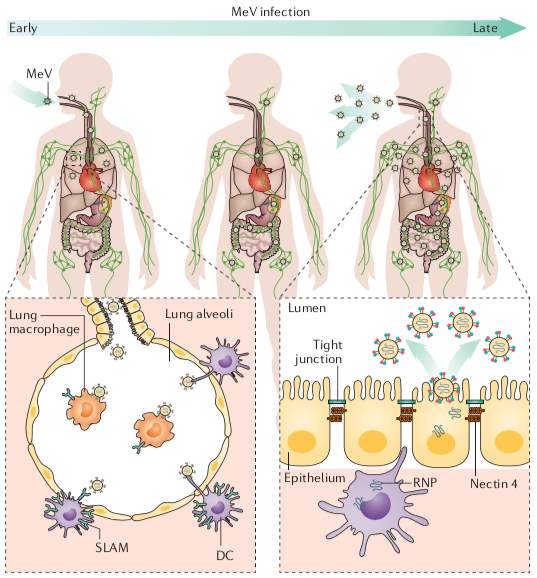
Figure 3. Measles virus infection and transmission (Rota et al., 2016, p. 6).
After MV enters into the respiratory tract, the virus infects alveolar macrophages or DCs by using SLAM. Infected macrophages or DCs transfer to the subepithelial cell layer and transmit the virus to epithelial cells by using nectin-4.
Based on biological characteristics as discussed above, it can be said that MV has some biological barriers in eradication and is highly contagious. Firstly, as measles is an airborne virus, it transmits via small virus particle sprayed into the air when individuals cough and even transports over long distances (Bloch et al., 1985; Racaniello, 2011). Thus, if individuals are exposed to the air, they can become easily infected through the respiratory tract. Secondly, MV is easy to bind with host cells when the virus enters respiratory tract because it has H protein binding to SLAM, moreover, MV is rapidly spread into various organs or tissues by H protein combining with nectin-4 (Laksono et al., 2016). Thirdly, MV has high stability on the surface, thus, it can survive for at least one hour in the airspace (Bloch et al., 1985). Therefore, owing to those biological barriers, the basic reproductive number (R0) of measles is higher estimating 12 to 18 than other infectious diseases, which implies that individuals with measles can transmit 12 to 18 other people on average in a totally susceptible population (Moss and Griffin, 2006).
Symptoms and immune responses
Approximately 10 days after MV infection, clinical signs appear with prodromal symptoms including fever, conjunctivitis and Koplik’s spots, followed by rash that continues 3 to 5 days (Figure 4). In general, individuals with measles are the most contagious starting from the last day of the incubation period through the prodromal and rash phase, which means that the incubation period is longer than the latent period, hence, infected people can be more transmittable as they are more likely to be unaware of their infection. During the prodromal phase, innate immune response starts with the production of natural killer cells or IFN-α and β (Moss and Griffin, 2006). In addition, adaptive immune response initiates with the manifestation of rash when CD4 and CD8 T cells move into the sites of virus replication, afterwards, virus clearance occurs. (Griffin, 2010). When looking at Figure 4, virus-specific CD8 T cells have a rapid activation, expansion and contraction. Although CD4 T cells response initiates simultaneously with CD8 T cells response, activation of CD4 T cells is extended. Virus-specific IgM used in the diagnosis of measles emerges with rash, which is followed by the prolonged synthesis of virus-specific IgG indicating prior exposure to measles (infection or immunisation).

Figure 4. Clinical symptoms and Immune responses (Griffin, D.E., 2010, p. 179).
Clinical symptoms begin with conjunctivitis, cough and Koplik’s spots after 10 days of infection, and rash emerges continuing 3 to 5 days. After CD 4 and CD 8 T cells appears for immune responses, IgM starts to produce with the emergence of rash, followed by the sustained synthesis of IgG. Immune suppression occurs during the acute disease and it continues long after recovery.
Most importantly, concerning immune responses, MV causes a temporary or profound immune suppression such as lymphopenia with decreasing CD4 and CD8 T cells and B cells during acute disease (Figure 4; de Vries et al., 2012), which can be a clinical burden because it leads to increased susceptibility to opportunistic infections that result in complications including otitis media, pneumonia and diarrhoea (Strebel and Orenstein, 2019). Particularly, infants, adults older than 20 years old, pregnant women, under-nourished children (vitamin A deficiency) are at an increased risk of complications from MV (ibid). Although the lymphopenia returns to the normal condition after patient recovery from fever and rash, immunological abnormalities continue long after at least a few weeks or even years. Thus, Mina et al. (2015) argue that opportunistic infections caused by immune suppression have an impact on increased mortality among children and may result in sustained mortality for 2 to 3 years after infection.
Diagnosis
Both detection of MV-RNA by reverse transcription PCR (RT-PCR) and detection of anti-MV IgM antibodies by enzyme immunoassay (EIA) are mainly used in the diagnosis of measles (Rota et al., 2016). Specifically, EIA is commonly used for laboratory confirmation of measles through serum samples collected 3 to 28 days after rash onset (Helfand et al., 1997) considering high sensitivity (83% to 89%; higher after the first week of rash onset) and specificity (95% to 100%) (Ratnam et al., 2000). Furthermore, EIA only requires a small sample (>20mL serum) and is rapid and simple to carry out by trained medical technicians (Bellini and Helfand, 2003). When MV isolation is impossible or unsuccessful, RT-PCR could be a more reliable diagnostic with samples collected through throat or nasal swabs, oral fluid, urine and peripheral blood mononuclear cells. It is because RT-PCR has higher sensitivity and specificity (94% and 99%) compared to EIA when samples are collected as soon as symptomatic before IgM antibodies are detectable (Roy et al., 2017). Additionally, RT-PCR has the further advantage which enables genotyping used for molecular epidemiology by tacking virus spreads and importations (WHO, 2018).
However, although typical cases are easily detected by EIA and RT-PCR during outbreaks, there are still limitations in the diagnosis of measles. Strebel and Orenstein (2019) demonstrate that roughly 25% of individuals tested within the first 72 hours after the appearance of rash, fails to detect IgM antibodies resulting in the problem of false-negative results. For instance, only 70% of confirmed cases within the first day after rash onset, detected IgM antibodies by CDC capture EIA (Helfand et al., 1997). Furthermore, false-positives arise from rheumatoid factor in samples because it can capture IgM component when performing EIA (Bellini and Helfand, 2003). Moreover, EIA requires a blood specimen and cold chain, therefore, individuals are less likely to have blood collected and it needs technically sophisticated equipment (ibid). Likewise, RT-PCR is challenging in sample collection because these samples should be collected within the first week of rash onset. Hence, as the timely sample collection of adequate quality for EIA and RT-PCR can be challenging to some clinicians who are less experienced in treating measles cases when patients present in clinics before rash onset or with less appearance of rash, the clinical diagnosis of measles is still problematic.
Conclusion
This essay has argued that although remarkable progress in reducing global incidence and mortality rates of measles has been made with a highly effective vaccination, MV still remains a cause of epidemics leading to global deaths and has some challenges such as MCV coverage, biological and clinical barriers, which can be obstacles to eradicate MV. Firstly, it is doubtful whether MCV coverage is still the most effective control measure in recent years because some countries with higher MCV2 coverage (95%) have increased incidence rates than those countries with lower MVC2 coverage (50%) from 2017 to 2019. Secondly, the biological characteristics of MV including F and H proteins, airborne virus and high stability in the airspace can be barriers that make MV more contagious indicating severe R0 (12 to 18). Thirdly, as MV has a longer incubation period than the latent period, infected people can be more transmittable to others. Additionally, opportunistic infections caused by immune suppression in measles cases can be a clinical burden that results in complications leading to increased mortality. Lastly, although RT-PCR and EIA are commonly used in the diagnosis of measles, they have clinical barriers regarding accurate diagnoses at rare intervals as those diagnostic tests should be conducted with adequate samples collected within a certain time (after rash onset), and EIA still has problems with false-negatives and positives. In conclusion, not only control measures including MCV coverage should be reassessed taking into account of the cause of the recent outbreaks including population movement and anti-vaxxers but also biological and clinical barriers should be considered in tandem with sufficient studies in order to eradicate measles throughout the world.
References
- Bellini, W.J. and Helfand, R.F. (2003). ‘The Challenges and Strategies for Laboratory Diagnosis of Measles in an International Setting’. The Journal of Infectious Diseases, 187 (1), pp. S283-S290. doi: 10.1086/368040.
- Bloch, A.B., Orenstein, W.A., Ewing, W.M., Spain, W.H., Mallison, G.F., Herrmann, K.L. and Hinman, A.R. (1985). ‘Measles Outbreak in a Pediatric Practice: Airborne Transmission in an Office Setting’. Pediatrics, 75 (4), pp. 676-683.
- Dabbagh, A., Laws, R.L., Steulet, C., Dumolard, L., Mulders, M.N., Kretsinger, K., Alexander, J.P., Rota, P.A. and Goodson, J.L. (2018). ‘Progress Toward Regional Measles Elimination — Worldwide, 2000–2017’. Morbidity and Mortality Weekly Report, 67 (47), pp. 1323-1329. doi: 10.15585/mmwr.mm6747a6.
- de Vries, R.D., McQuaid, S., van Amerongen, G., Yüksel, S., Verburgh, R.J., Osterhaus, A.D.M.E., Duprex, W.P. and de Swart, R.L. (2012). ‘Measles Immune Suppression: Lessons from the Macaque Model’. PLoS Pathogens, 8 (8), p. e1002885. doi: 10.1371/journal.ppat.1002885.
- Griffin, D.E. (2010). ‘Measles virus-induced suppression of immune responses’. Immunological Reviews, 236 (1), pp. 176-189. doi: 10.1111/j.1600-065X.2010.00925.x.
- Griffin, D.E. (2013) Fields Virology. 6th edn. Edited by David M. Knipe and Peter M. Howley. Philadelphia, PA, USA: Lippincott Williams & Wilkins.
- Hashiguchi, T., Maenaka, K. and Yanagi, Y. (2011). ‘Measles Virus Hemagglutinin: Structural Insights into Cell Entry and Measles Vaccine’. Frontiers in Microbiology, 2, p. 247. doi: 10.3389/fmicb.2011.00247.
- Helfand, R.F., Heath, J.L., Anderson, L.J., Maes, E.F., Guris, D. and Bellini, W.J. (1997). ‘Diagnosis of Measles with an IgM Capture EIA: The Optimal Timing of Specimen Collection after Rash Onset’. Journal of Infectious Diseases, 175 (1), pp. 195-199. doi: 10.1093/infdis/175.1.195.
- Heywood, A.E. (2018). ‘Measles: a re-emerging problem in migrants and travellers’. Journal of Travel Medicine, 25 (1), pp. 1-2. doi: 10.1093/jtm/tay118.
- Javelle, E., Colson, P., Parola, P. and Raoult, D. (2019). ‘Measles, the need for a paradigm shift’. European Journal of Epidemiology, 34 (10), pp. 897-915. doi: 10.1007/s10654-019-00569-4.
- Laksono, B.M., de Vries, R.D., McQuaid, S., Duprex, W.P. and de Swart, R.L. (2016). ‘Measles Virus Host Invasion and Pathogenesis’. Viruses, 8 (8), p. 210. doi: 10.3390/v8080210.
- McLean, H.Q., Fiebelkorn, A.P., Temte, J.L. and Wallace, G.S. (2013). ‘Prevention of Measles, Rubella, Congenital Rubella Syndrome, and Mumps, 2013: Summary Recommendations of the Advisory Committee on Immunization Practices (ACIP)’. Morbidity and Mortality Weekly Report, 62 (4), pp. 1-34.
- Mina, M.J., Metcalf, C.J.E., de Swart, R.L., Osterhaus, A.D.M.E. and Grenfell, B.T. (2015). ‘Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality’. Science, 348 (6235), pp. 694-699. doi: 10.1126/science.aaa3662.
- Moss, W.J. and Griffin, D.E. (2006). ‘Global measles elimination’. Nature Reviews Microbiology, 4 (12), pp. 900-908. doi:10.1038/nrmicro1550.
- Orsoo, O., Saw, Y.M., Sereenen, E., Yadamsuren, B., Byambaa, A., Kariya, T., Yamamoto, E. and Hamajima, N. (2019). ‘Epidemiological characteristics and trends of a Nationwide measles outbreak in Mongolia, 2015–2016’. BMC Public Health, 19 (1), p. 201. doi: 10.1186/s12889-019-6511-0.
- Phadke, V.K., Bednarczyk, R.A., Salmon, D.A. and Omer, S.B. (2016). ‘Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States: A Review of Measles and Pertussis’. JAMA, 315 (11), pp. 1149-1158. doi: 10.1001/jama.2016.1353.
- Racaniello, V. (2011). ‘An Exit Strategy for Measles Virus’. Science, 334 (6063), pp. 1650-1651. doi: 10.1126/science.1217378.
- Ratnam, S., Tipples, G., Head, C., Fauvel, M., Fearon, M. and Ward, B.J. (2000). ‘Performance of Indirect Immunoglobulin M (IgM) Serology Tests and IgM Capture Assays for Laboratory Diagnosis of Measles’. Journal of Clinical Microbiology, 38 (1), pp. 99-104.
- Rota, P.A., Moss, W.J., Takeda, M., de Swart, R.L., Thompson, K.M. and Goodson, J.L. (2016). ‘Measles’. Nature Reviews Disease Primers, 2 (1), pp. 1-16. doi: 10.1038/nrdp.2016.49.
- Roy, F., Mendoza, L., Hiebert, J., McNall, R.J., Bankamp, B., Connolly, S., Lüdde, A., Friedrich, N., Mankertz, A., Rota, P.A. and Severini, A. (2017). ‘Rapid Identification of Measles Virus Vaccine Genotype by Real-Time PCR’. Journal of Clinical Microbiology, 55 (3), pp. 735-743. doi: 10.1128/JCM.01879-16.
- Strebel, P.M. and Orenstein, W.A. (2019). ‘Measles’. New England Journal of Medicine, 381 (4), pp. 349-357. doi: 10.1056/NEJMcp1905181.
- Tahara, M., Bürckert, J.P., Kanou, K., Maenaka, K., Muller, C.P. and Takeda, M. (2016). ‘Measles Virus Hemagglutinin Protein Epitopes: The Basis of Antigenic Stability’. Viruses, 8 (8), p. 216. doi: 10.3390/v8080216.
- World Health Organization. (2012). Global measles and rubella strategic plan: 2012-2020. Geneva: WHO Press. Available at: https://www.afro.who.int/sites/default/files/2017-06/9789241503396_eng.pdf (Accessed: 06 Jan 2020).
- World Health Organization. (2017). ‘Measles vaccines: WHO position paper – April 2017’. Wkly Epidemiol Rec, 92 (17). pp. 205-228. Available at: https://apps.who.int/iris/bitstream/handle/10665/255149/WER9217.pdf?sequence=1 (Accessed: 06 Jan 2020).
- World Health Organization. (2018). Immunization, Vaccines and Biologicals: Manual for the Laboratory-based Surveillance of Measles, Rubella, and Congenital Rubella Syndrome. Available at: https://www.who.int/immunization/monitoring_surveillance/burden/laboratory/manual/en/ (Accessed: 06 Jan 2020).
- World Health Organization. (2019a). Immunization, Vaccines and Biologicals: New measles surveillance data for 2019. Available at: https://www.who.int/immunization/newsroom/measles-data-2019/en/ (Accessed: 06 Jan 2020).
- World Health Organization. (2019b). Ten threats to global health in 2019. Available at: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 (Accessed: 06 Jan 2020).
Cite This Work
To export a reference to this article please select a referencing style below:

