Introduction
A subarachnoid haemorrhage (SAH) is when blood enters into the subarachnoid space between the pial and arachnoid membrane (FIGURE 1). SAH may be to congenital or acquired with the most common cause being intracranial aneurysms which account for well over 75% of cases. Although classified as a type of stroke, SAH can differ from the other types of strokes in both presentation and management (Smith et al 2007).
SAH accounts for a small percentage of all strokes (5%) and affects around 10 per 100 000 of the UK population per year. The condition is extremely serious with a Mortality is around 50%. A large cohort die before even reaching hospital. A third of survivors (30%) are dependent on long-term care and a significant proportion suffers cognitive impairment sufficient to affect their quality of life (Smith et al 2007) (Diringer et al 2009).
SAH requires a multi-disciplinary approach to management in a specialist centre with treatment principally directed towards minimizing secondary brain injury and preventing / treating systemic complications.
In 2011, the Neuro Critical Care Society issued consensus guidelines for the critical care management of aneurysmal SAH with the aim of improving outcomes (Diringer et al 2011)
This case study discusses a patient who presented with SAH secondary to a ruptured cerebral aneurysm. This study will include clinical presentation, relevant investigations and an analysis of care and treatment used. Finally this study will discuss the pathophysiology of this condition and review current literature on management of such patients through a multidisciplinary approach.
Clinical case
Mrs. X was a 62 years old lady who presented to an emergency department at a peripheral hospital. She arrived with her husband with sudden onset of severe headache. Her husband reported she had brief loss of consciousness and mild confusion. On arrival, her vital signs were recorded as stable. Her airway was clear. She was slightly tachypneoic (Respiratory rate 20) with oxygen saturations of 95% on room air. Her blood pressure was 158/ 70 and heart rate 75 and regular. Temperature 36.9.
Mrs. X was assessed neurologically using the Glasgow coma scale (GCS). Her pupils were size 3 and were equal and reacting on both sides. She would open her eyes to voice, obey commands, but was confused. She could move all limbs equally with good strength. Her GCS at presentation was recorded as 13 (E 3, V 4, M 6). (Brennan et al 2018)
Her pain score from her headache was recorded as 7/10, measured on a scale of 0 being nothing and 10 being the worst ever pain experienced.
Mrs. X had no significant past medical history. She was normally independent. She was a regular smoker. (>20 day)
Blood results taken at presentation were normal including blood glucose.
A CT head scan was performed which showed an anterior communicating artery aneurysm with a small subarachnoid bleed. This prompted transfer to a specialist neurosurgical centre for further review and management.
Mrs. X was transferred to a high dependency unit (HDU) in a tertiary referral centre. On arrival she was connected to a monitor and all vital signs were recorded. She continued to maintain her own airway and have mild tachypnea. She was started on 2 litres of oxygen via nasal cannula as her oxygen saturations had dipped to 92% and she remained mildly confused. She remained haemodynamically stable but it was decided an arterial line was needed to closely monitor blood pressure and to obtain arterial gases. An arterial blood sample was taken to check metabolic status, electrolytes, lactate and blood glucose. Lactate was found to be marginally elevated. All other lab bloods were repeated and a group and save was sent incase further bleeding occurred or surgical intervention was needed. A urinary catheter was inserted to monitor fluid balance and intra venous Hartmann’s was commenced at a maintenance input of 3 litres over 24 hours. Her GCS remained at 13/15 due to mild confusion/ drowsiness.
Mrs. X headaches remained excruciating despite an initial dose of IV Morphine so was prescribed and given Oromorph pro re nata (prn) should she need it along with anti-emetics. Regular paracetamol was prescribed for further pain relief and Nimodipine 60 milligrams (mg), four hourly to reduce vasospasm.
Mrs. X had a full secondary survey. Pressure areas were checked and cot sides raised to prevent falls. She was measured for and given anti-embolic stockings.
Neurosurgical review was promptly undertaken after admission to HDU. CT scans were reviewed and confirmed a small subarachnoid bleed from an anterior communicating aneurysm. Instructions were for close neurological monitoring overnight with maintenance of blood pressure within a range of 140–160 mmHg systolic. A CT angiogram and coiling was planned for the following morning. If Mrs. X’s GCS deteriorated at all overnight she was for further neurosurgical review and repeat CT head.
Endovascular coiling using platinum coils was performed in the morning by the interventional neuro-radiologists. There were no peri-procedural complications or postoperative vasospasm. The patient demonstrated no neurological deficits on return to critical care.
Close haemodynamic monitoring and neurological observations were carried on post operatively to detect any signs of neurological deterioration. Bed rest sat slightly upright was advised for 4 hours post operatively. Observation of the puncture site was observed for signs of bleeding, swelling or infection. Foot pulses were also checked 2 hourly along with skin colour to the feet and toes. Nimodipine 60mg was continued 4 hourly and prescribed for a total of three weeks. Aperients were prescribed along with prophylactic enoxaparin until fully mobile. Mrs. X was discharged from hospital 6 days post procedure with no recorded inpatient complications.
Discussion
Acute subarachnoid hemorrhage (SAH) is a life threatening condition that affects multiple organ systems, not just the brain. Although more lives are saved than ever before, this entity is still associated with considerable morbidity and mortality (DeSouza et al 2015).
The most common cause of acute SAH is a ruptured cerebral aneurysm. An “aneurysm” is a bulging, weakened area of an artery wall, which results in a widened or ballooning area. There are three common types: (1) sacular shaped, like a berry with a narrow stem, such as Mrs X’S: 2) fusiform, which bulges out on all sides and (3) dissecting, which results from a tear along the inner artery wall (FIGURE 2). Cerebral aneurysms tend to occur within the circle of Willis (FIGURE 3). The vast majority of cerebral aneurysms are located in the anterior arterial circulation (>80%) and less than 20% in the posterior circulation. (Beck et al, 2006).
Up to quarter (10-25%) of all patients with acute SAH either die straight after the bleed or before they arrive in hospital. A third require permanent nursing care, and almost a similar number are able to return to independent living (DeSouza et al 2015). The clinical outcome depends on multiple factors, including the severity of the initial bleed, the patient’s initial condition, re-bleeding, and the severity of delayed cerebral ischemia (DCI).
(Lieshout et al. 2017) showed that delayed transfer to a neurosurgical unit significantly worsens mortality. Mrs. X was promptly referred and sent to a specialist centre therefore reducing her risk of mortality and potentially morbidity.
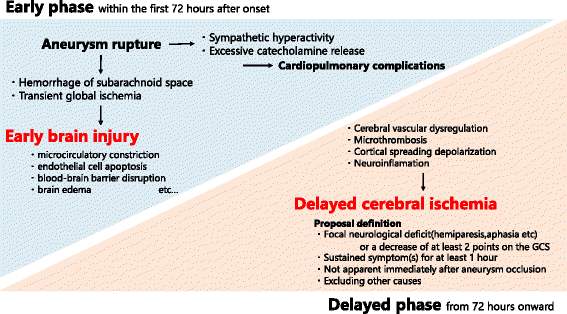
Early brain injury (EBI) and DCI are well-studied mechanisms that develops in SAH patients within the first 72 hours (EBI) and out to 14 days after the hemorrhage (Lawton et al 2017). This window of opportunity is possibly where nursing and medical intervention can be most effective.
There are a number of physiological and pathological processes that contribute to the development of EBI and DCI. In the early phase, aneurysm rupture leads to transient global ischemia with increasing intracranial pressure. This results in increased resistance to cerebral blood flow. There is decreasing cerebral perfusion pressure, and reduced cerebral blood flow. There is a build up of toxins from the initial subarachnoid hemorrhage. These mechanisms induce their own detrimental effect; such as constriction of the microcirculation, cell death, disruption of the blood–brain barrier, and an inflammatory cascade with progressive intra-cranial oedema, (FIGURE 4).
DCI usually develops in 30% of SAH patients between 4 days to 2 weeks after the hemorrhage. Cerebral vasospasm was thought to be the main cause of DCI however a clear picture remains unknown. Proposed mechanisms include loss of cerebral vascular homeostasis, microcirculatory dysfunction, microthrombi, inflammation and abnormal electrical signal generation within the brain.(Geraghty et al 2017) (Cook et al 2004).
The clinical manifestations of this cerebral dysregulation in SAH include signs of high intracranial pressure, (nausea, vomiting, reduced consciousness or coma), signs of meningeal irritation (photophobia, severe headache), focal neurological deficits and seizure activity.
Increased sympathetic activity (release of catecholamines) can lead to cardiorespiratory complications from subtle ECG abnormalities to myocardial infarction. Elevated catecholamine secretion SAH can lead to pulmonary hypertension and oedema. Electrolyte disturbances are a common finding, occuring in approximately 33% of patients. Salt wasting syndromes such as SIADH resulting in hypovolaemia and further intra-cerebral hypo-perfusion. (Rose et al 2011). Dysnatremia management of both hyponatremia and hypernatremia are closely observed to maintain optimum levels. Mrs. X’s sodium as with other electrolytes was monitored closely and remained stable. But often patients can present with hypernatremia initially and hyponatremia several days later (Okazaki 2017). It is difficult to prove that that deranged sodium can have an impact on outcome and further studies have been suggested (Okazaki 2018).
Mrs. X blood glucose was stable between 8–10 the center’s suggested parameters so no intervention was required. Hyperglycemia is frequently observed in SAH patients and is associated with poor outcomes (Steiner et al 2013). The European Stroke Organization guidelines recommend “that hyperglycemia with blood glucose > 10 mmol/L should be treated.” However, there is no evidence supporting this cutoff value. Similarly, Naidech et al 2010 observed, “hypoglycemia in SAH patients is associated with poor outcomes”. His retrospective observational study found that over half of SAH patients with glucose < 8 mmol/L had unfavorable outcomes. As such close glycaemic control is advised in these patients.
Until clipping or coiling has secured the aneurysm, all patient specific parameters must be regularly monitored (eg. cardiac monitoring, early warning scores, blood pressure). Neurological status must be documented (eg. conscious level, GCS, pupillary response, and any observable neurologic deficits). Deterioration can occur rapidly. Mrs. X had all her vital signs and full neurological assessment hourly overnight, which is very obtrusive on rest but vital in detecting deterioration in condition. She had no requirement or means of intracranial pressure monitoring.
Because aneurysmal re-rupture carries 70–90% mortality, all risk factors that can promote re-rupture need to be thoroughly and continuously addressed.
Mrs. X was very stable in her respiratory status not requiring invasive ventilation, so for her the need for regular CO2 monitoring was not required. This is a factor, which should be monitored closely and maintained in ventilated patients.
Blood pressure control is extremely important. In particular rapid increases in blood pressure. Keeping the systolic blood pressure below 140 mmHg is recommended. Triple H therapy – Hypertension, hypervolemia and haemodilution (HHH) has been widely used to treat vasospasm but no evidence has supported this (Meyer et al 2011). Induced hypertension and hypervolemia has been recommended to maintain hemodynamic and fluid stability of patients (Francoeur et al 2016). The guide for Mrs. X was to maintain a systolic blood pressure of 140 – 160 and fluid input of 3L to maintain good cerebral perfusion and reduce the risk of vasospasm. Luckily she was within these parameters overnight so did not need any further intervention. If she did experience hypotension the plan would have been to firstly increase the intravenous fluid rate and volume. Had this not been successful her prescription of Nimodopine would have been changed to 30mg two hourly instead of 60mg 4 hourly. This is sometimes more effective in reducing the side effect of hypotension but continuing with the drug for its benefits. A final plan would have then been to add an inotrope such as noradrenaline. If hypertension was experienced, issues such as pain and agitation would need to be addressed first before using considering antihypertensive medication such as intra venous labetalol.
Any conceivable cause for stress must be recognized and/or treated if possible, i.e., pain, anxiety or agitation. In particular painful procedures / invasive monitoring must be carefully considered (eg. central venous lines, repeated cannulation as well as moving and handling, in an un-sedated patient). As in Mrs. X case the medical staff continued on with siting an arterial line as they felt like the benefits of invasive blood pressure readings and easy access to blood samples without causing any further discomfort outweighed the disadvantages.
Mrs. X did complain of an excruciating headache so (IV) morphine was given followed by prn Oromorph, which kept her comfortable and settled overnight. This was very important to keep her other parameters within range and reduce her risk of DCI.
Following neurosurgical review it was decided the best treatment plan would be for the patient to go for coiling of the aneurysm the following morning.
The mainstay of treatment for acute SAH remains surgical clipping versus endovascular coiling. There is contention between which intervention is superior. To date there have been four prospective randomized controlled trials (RCT). The two most relevant trials to guiding clinic practice are the International Subarachnoid Aneurysm Trial (ISAT) and the Barrow Ruptured Aneurysm Trial (BRAT). On the basis of these study results there as been a shift in treatment from clipping to coiling around the U.K. (Molyneux et al 2005), (Molyneux et al 2015),
The largest study so far (ISAT) was carried out on 2143 patients across 42 neurosurgical centres mainly across the UK and Europe. Patients were randomly assigned to neurosurgical clipping (1070) or endovascular coiling (1073). The primary outcome was death or dependence at 1 year (defined by a modified Rankin scale of 3-6). Secondary outcomes included rebleeding from the treated aneurysm and risks of seizures were also looked at (Molyneux et al., 2005)
(ISAT) showed “favourable results for coiling in almost all outcome measures” (eg. Glasgow Outcome Scale and modified Rankin Scale. (Molyneux et al., 2005)
Unfortunately this study was stopped prematurely simply because early results favoured coiling at 1 year-follow-up. As such, these findings have generalized practice in acute SAH in some parts of the globe. This is despite that fact that on further analysis of the longer term outcomes and other randomized controlled trials revealed that there really is no superiority of coiling over clipping in terms of longer term survival rate – when studied over 3-5 years. (Rafael Martinez-Perez et al 2019; Spetzler et al., 2015).
The (BRAT) study was a 6-year RCT that compared the safety and efficacy of surgical clipping vs endovascular coil embolization in patients presenting with (SAH) from a ruptured aneurysm. 500 patients were selected which resulted in 408 patients undergoing clipping (209 assigned) or coiling (199 assigned). (Spetzler et al 2015).
Although (BRAT) was statistically underpowered to detect small differences, the results suggested little differences in outcomes between the 2 treatments for anterior circulation aneurysms. Aneurysm obliteration rates in (BRAT) were significantly lower and retreatment rates significantly higher in the patients undergoing coiling than in those undergoing clipping. Enough questions were raised about the relative benefits of the 2 treatment modalities to warrant further well-designed randomized trials (Spetzler et al 2015).
Proponents of surgical clipping state advantages of this technique include, the ability to survey complex anatomy of the aneurysm. Also, handling this anatomy can be more informative than any angiographic image (Hademenos et al 1998). Neurosurgeons also feel microsurgical-clipping holds better place than coiling due to lower rates of recurrence and re-bleeding.
Epidemiological studies have claimed that patients with ruptured and un-ruptured cerebral aneurysms treated with clipping are more often discharged to long-term facilities. This may infer that endovascular coiling is much safer, leading to faster recovery and reduced mortality and morbidity. However, there may be a selection bias here where more complex or larger aneurysms are preferentially surgically clipped may not be amenable to endovascular coiling. (Brinjikji et al. 2011).
In choosing the best treatment option considerations of access, age, shape, size and rupture status need to be taken in to account. The management, rather than choosing one over the other should be choosing the treatment, which gives the patient maximum benefit with the least complications (Sharma et al 2019).
Conclusion
SAH from a cerebral aneurysm is a very serious condition affecting many patients. It can present at any time with either no or distinct symptoms of a severe headache, nausea and altered neurological state. Symptoms can present just prior to SAH as a warning sign or post SAH. It is vital to recognise such symptoms and seek urgent medical review and transfer to a specialist neurological centre, which can improve outcome and reduce mortality.
Once SAH has been confirmed a review by a neurosurgeon needs to be sought and a plan of care made. Close observation of neurological status needs to be observed to reduce the risk of DCI and identify any signs of deterioration in condition. Haemodynamic observations including blood pressure should be regularly monitored and maintained within a defined range. Fluid balance should be monitored and maintained to a level of hypervolemia. Laboratory bloods including electrolytes and blood glucose should be monitored closely and corrected accordingly to maintain levels. Other stress factors should be addressed such as the need for invasive procedures, pain, anxiety and sleep to maintain patient outcome and comfort.
There continues to be ongoing debate about best treatment between coiling and clipping. Following the ISAT and Brat study there seemed to be a shift towards coiling. It was seen to carry less risk factor than open surgery like clipping. However surgery offered the advantage of a close view of anatomy of wide neck aneurysm, which has the risk of coil migration and arterial occlusion. Ultimately no difference is noted in either procedure beyond one year and the general consensus is that timing of treatment is more important than choice of treatment.
References
(1) Beck J, Rohde S, Berkefeld J, Seifert V, Raabe A. Size and location of ruptured and unruptured intracranial aneurysms measured by 3-dimensional rotational angiography. Surg Neurol. 2006 Jan;65(1):18-25; discussion 25-7. PMID 16378842.
(2) Brennan PM, Murray GD, Teasdale GM: Simplifying the use of prognostic information in traumatic brain injury. Part 1: The GCS-Pupils score: an extended index of clinical severity. J Neurosurg:2018
(3) Cook NF (2004) Subarachnoid haemorrhage and vasospasm: using physiological theory to generate nursing interventions. Intensive Critical Care Nurse 20(3): 163–73
(4) D‘Souza S: Aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 2015; 27: 222–40.
(5) Diringer MN, Bleck TP, Hemphill JC et al. on behalf of the Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid haemorrhage: recommendations from the Neurocritical Care Society Multidisciplinary Consensus Conference. Neurocrit Care 2011; 15: 211–40
(6) Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after
subarachnoid hemorrhage. Crit Care. 2016;20:277.
(7) Geraghty JR, Testai FD. Delayed cerebral ischemia after subarachnoid hemorrhage: beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep. 2017;19:50
(8) Hademenos GJ, Massoud TF, Turjman F, Sayre JW: Anatomical and morphological factors correlating with rupture of intracranial aneurysms in patients referred for endovascular treatment. Neuroradiology 1998; 40: 755–60.
(9) Kundra S, Mahendru V, Gupta V, Choudhary AK: Principles of neuroanesthesia in aneurysmal subarachnoid hemorrhage. J Anaesthesiol Clin Pharmacol 2014; 30: 328–37.
(10) Lawton MT, Vates GE. Subarachnoid hemorrhage. N Engl J Med. 2017;377: 257–66.
(11) Lieshout V , Bruland I, Fischer I, et al.: Time is life. Increased mortality of patients with aneurysmatic subarachnoid hemorrhage caused by prolonged transport time to a high-volume neurosurgical unit. Am J Emerg Med 2017; 35: 45–50.
(12) Meyer R, Deem S, Yanez ND, Souter M, Lam A, Treggiari MM. Current practices of triple-H prophylaxis and therapy in patients with subarachnoid hemorrhage. Neurocrit Care. 2011;14:24–36.
(13) Molyneux AJ, Kerr RS, Yu LM, et al.: International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366: 809–17.
(14) Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS: The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet 2015; 385: 691–7.
(15) Naidech AM, Levasseur K, Liebling S, Garg RK, Shapiro M, Ault ML, et al.
Moderate hypoglycemia is associated with vasospasm, cerebral infarction,
and 3-month disability after subarachnoid hemorrhage. Neurocrit Care.
2010;12:181–7.
(16) Okazaki T, Hifumi T, Kawakita K, Shishido H, Ogawa D, Okauchi M, et al. Target serum sodium levels during intensive care unit management of aneurysmal subarachnoid hemorrhage. Shock. 2017;48:558–63.
(17) Okazaki T,Kuroda Y Aneurysmal subarachnoid hemorrhage: intensive care for improving neurological outcome (2018) Journal of Intensive Care. Vol 6, Article number: 28
(18) Rafael Martínez-Pérez, MD, PhD,1,2* Natalia Rayo,3 Agustín Montivero,2and Jorge Marcelo Mura2The “Brain Stress Timing” phenomenon and other misinterpretations of randomized clinical trial on aneurysmal subarachnoid haemorrhage. Neural Regen Research. 2019 Aug; 14(8): 1364–1366.
(19) Rose MJ: Aneurysmal subarachnoid hemorrhage: An update on the medical complications and treatments strategies seen in these patients. Curr Opin Anaesthesiol 2011; 24: 500–7.
(20) Sharma, Rajan Kumar ; Yamada, Yasuhiro ; Kawase, Tsukasa ; Kato, Yoko To clip or coil? Proposal of individual decision making. Interdisciplinary Neurosurgery: Advanced Techniques and Case Management, September 2019, Vol.17, pp.124-128
(21) Smith M. Intensive care management of patients with subarachnoid haemorrhage. Curr Opin Anaesthesiol 2007; 20: 400–7
(22) Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Russin JJ, Partovi S, Nakaji P, Wallace RC. Journal of Neurosurgery. 2015 Sep;123(3):609-17. doi: 10.3171/2014.9.JNS141749. Epub 2015 Jun 26.The Barrow Ruptured Aneurysm Trial: 6-year results.
(23) Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European Stroke Organization guidelines for the management of intracranial aneurysms and
subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35:93–112.
Figures
FIGURE 1 – Layer of the Meninges

FIGURE 2 – 3 Common Types of Cerebral Aneurysms
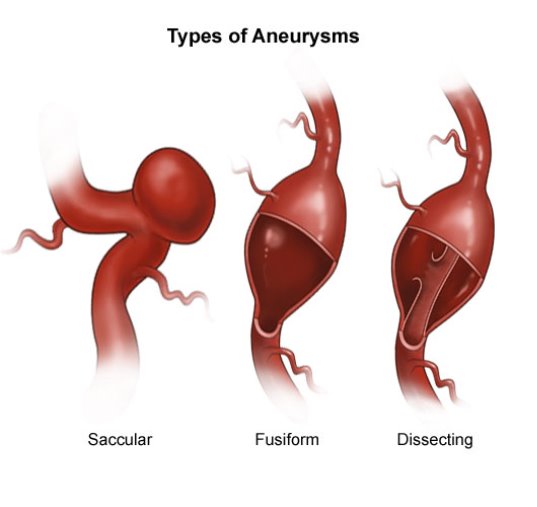
FIGURE 3 – Arterial Supply to the Brain and the Circle of Willis

FIGURE 4 – Early Brain Injury and Delayed Cerebral Ischaemia – Pathophysiology
Aneurysmal subarachnoid hemorrhage: intensive care for improving neurological outcome
Okazaki T, Kuroda Y (2018) Journal of Intensive Care. Vol 6, Article number: 28
Cite This Work
To export a reference to this article please select a referencing style below:

