Abstract
Within the following report various different studies will be analysed to assess whether Polycyclic Aromatic Hydrocarbons e.g. Benzo[a]pyrene cause Breast Cancer. PAHs are ubiquitous environmental pollutants formed during the incomplete combustion of organic materials. PAHs contain carcinogenic properties and can exert their effects on humans by ingestion, inhalation and adsorption. Although the exact etiology of breast cancer is unknown, various case-control studies have been carried out that prove a direct correlation between areas with high levels of PAH concentration and the incidence of breast cancer. It’s also been shown that there was a higher level of BaP-DNA adducts in cancer patients than controls suggesting they’ve a role to play in the implication of the disease. It has also been seen that smoking causes human bronchial cells to produce stress-induced unregulated cRNA transcripts. These transcripts can also be seen in breast cancer cell lines, therefore suggesting that smoking can up regulate ncRNAs in breast cancer. Several other rodent in-vitro and in-vivo studies have been done to show how PAHs may implicate breast cancer. However not all are directly relevant to human subjects as humans would not be exposed to such high concentrations of exposure, or exposure at regular intervals. Therefore, further studies must be done to confirm if PAHs directly implicate breast cancer.
Introduction
Polycyclic Aromatic Hydrocarbons (PAHs), are a hydrocarbon – which is an organic compound comprised of two or more fused benzene rings arranged in various different configurations. (Kim et al. 2013)
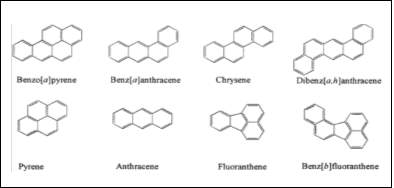
Figure 1:This figure shows the structure of common PAHs found in the environment. (Kim et al. 2013)
PAHs are uncharged nonpolar molecules found in coal and tar, but also they’re ubiquitous environmental pollutants and are formed during incomplete combustion of organic materials. (Abdel-Shafy and Mansour 2016)
Many PAHs contain mutagenic toxic and carcinogenic properties. PAH’s were one of the first atmospheric pollutants designated as a suspected carcinogen. Although the health effects of different PAHs differ, they’re noted to be of great concern due to their highly adverse and extreme health effects on humans. (Abdel-Shafy and Mansour 2016)
Governmental Bodies such as the European Commission, NIOSH and the US EPA are monitoring conditions, and enforcing legislation around PAHs in air soil and water. For example the European Commission has restricted concentrations of some specific carcinogenic PAHs in consumer products that contact the skin or mouth. A PAH which is of concern that I will be discussing throughout is benzo(a)preyene. (Kim et al. 2013)
Benzo(a)preyene (BaP), with the chemical structure C20H12, is a polycyclic aromatic hydrocarbon which is found ubiquitous in nature, coal and tar. This specific PAH is found in smoked and grilled meats, making it a PAH of particular concern for humans. It is also classified as a group 1 carcinogen by the IARC. (Kim et al. 2013)
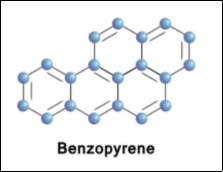
Figure 2: This figure shows the molecular structure of Benzopyrene (BaP) this is the most common PAH and is an ultimate carcinogen.(Lee and Shim 2007)
There is also a lot of studies and research suggesting that Benzo(a)preyene, along with other PAHs has a direct effect on the incidence of Breast Cancer. PAHs are a severe Endocrine Disrupters (EDs), which therefore can potentially cause negative hormone responses, resulting in Breast Cancer. (Kim et al. 2013)
Within my report I will be discussing:
- How PAHs were originally implicated in Breast Cancer
- The mechanism of exposure of PAHs
- Cohort and case control studies which have been used to implicate PAHs in Breast Cancer.
- The likely mechanism in which PAHs exerts their effect
- How the toxicants effects may be modified by interaction with other agents or how it reacts with the human genome to produce differing consequences for different genotypes.
- How PAHs were originally implicated in Breast Cancer
The exact etiology of Breast Cancer is unknown. There are of course a number of risk factors for the implication of breast cancer such as age, weight, genetics, race, smoking, exposure to radiation and diet.(Kim et al. 2013) Many carcinogens have been identified that are produced as a consequence of industrial activities, and traffic emissions.(Abdel-Shafy and Mansour 2016) And as stated, many carcinogens are speculated to implicate breast cancer such as BaP. As of yet there is no direct evidence to fully prove that PAHs of any kind can breast cancer.
However originally, it was Morris and Seifter in 1992 that suggested that the PAH emissions and the incidence of breast cancer in women were linked. The geographic locations of breast cancer cases, and also locations of high hydrocarbon residue levels were plotted. It was proven that these locations coincide. Therefore originally suggesting that PAHs implicate Breast cancer.(Morris and Seifter 1992)
- The goal of this paper was to access the role of aromatic hydrocarbons in the genesis of breast cancer. It was previously known that epidemiological markers of breast cancer can include endocrine related phenomena, and the exposure of the breast to X-Radiation. However, polycyclic aromatic hydrocarbons had not yet been proven to be implicated in human mammary carcinogens. Upon exploration of the role of breast tissue, it was seen that the breasts are particularly susceptible to aromatic carcinogenesis. Since the breasts are largely composed of fat, PAHs can be harboured easily within the tissue. These hydrocarbons are stored and metabolised into carcinogenic metabolites. Ductal cells then become target cells for carcinogenesis as they concentrate the metabolites. It was discovered that human exposure to PAH metabolites induce and promote altered DNA by mechanisms described as increased intracellular pro-oxidant production as well as direct adduction to DNA. A unitary model for mammary carcinogenesis in humans was therefore hypothesised. If this hypothesis was proven to be correct it would account for the increase in breast cancer incidence in industrialised countries, and potentially suggest modifications to be made to inhibit PAH induced mammary carcinogenesis. (Morris and Seifter 1992)
Similarly, in more recent years there was an ecological study carried out in the US in 2015. This study was done to analyse the incidence of breast cancer in relation to ambient PAH emission concentration in varying counties within the US. This study agreed with Morris and Seifters hypothesis, and it was found that highly industrialised areas shared a higher incidence of breast cancer. (Stults and Wei 2018)
- Data regarding PAH concentration was obtained by US EPA, and county-level data on the incidence of breast cancer was obtained. A linear regression was performed using SPSS 23 software for Windows to analyse the association between PAH emissions and breast cancer incidence. The data was refined in various ways. Data from unreliable county sources i.e Kalawao County of the Hawaii region were excluded. Furthermore Alameda, Contra Costa, Marin, San Francisco, and San Mateo counties in the San Francisco–Oakland region were also omitted due to the fact they had inaccurate PAH emission data. Womens breast cancer cases were also assessed and refined by age/family history/smokers and non-smokers etc. The more industrialized metropolitan regions, San Francisco–Oakland, Connecticut, Atlanta, Detroit, and Seattle, had a definite higher incidence of breast cancer, as compared to less industrialized regions, Hawaii, Iowa, New Mexico, and Utah. This proves that PAH exposure from ambient air could play a role in the increased breast cancer risk among women living in urban areas of the US. (Stults and Wei 2018)
Various other cross sectional studies were shown that PAHs may implicate breast cancer in animals such as rodents, however as stated the exact etiology of breast cancer for humans is still unknown. (Korsh et al. 2015)
- A cross sectional study was done in 1996 in America where DNA-adducts of breast cancer patients undergoing a mastectomy were analysed, and noncancer patients undergoing reduction mammoplasty were to serve as a control. It was found in this study that environmental carcinogen exposure(namely benzo(a)pyrene), found in cigarette smoke, may be associated with the etiology of human breast cancer. The results showed that while aromatic DNA adducts of some kind were found in all cases, the concentration of them within cancer patients compared to controls was significantly higher. Furthermore it was found that benzo(a)pyrene DNA adducts were found in 41% of breast cancer patients, however in noncancer patients there was no evidence of benzo(a)pyrene DNA adducts. Therefore this implicates that benzo(a)pyrene could potentially cause breast cancer. (Rundle et al. 2000)
- The mechanism of exposure of PAHs
There are three mechanisms of exposure of PAHs that effect humans. These include; Inhalation, Absorption, and Ingestion. (Baird et al. 2005)
- Inhalation is the most common mechanism of exposure of PAHs. They’re ubiquitous in air and therefore are easily inhaled, especially in urban areas where the concentration is higher. (Baird et al. 2005)
- Ingestion is another common mechanism of exposure, PAHs are commonly found in processed/charred/grilled/smokes meats. PAHs are also readily deposited on arable land therefore contaminating crops which humans consume. PAHs are highly lipid soluble and therefore readily absorbed from the gastrointestinal tract of mammals. (Abdel-Shafy and Mansour 2016, VanRooij et al. 1993)
- Absorption can also occur especially whereby people work within oil/tar industries and are constantly in contact with PAHs within coal/tar. (VanRooij et al. 1993)
- Cohort and Case Control studies on how PAHs cause Breast Cancer
Case Control Studies
A case control study is a study in which patients with a certain disease/outcome of interest (cases) are compared to patients do not have this disease/outcome of interest (control). These studies are crucial to examine and carry out when deciphering whether a certain chemical can implicate a disease, as it is easy to compare and contrast results. (The Himmelfarb Health Sciences Library, 2011) There has been many different case control studies done on how PAHs may influence breast cancer. However none of the studies have been conclusive, all need further study to be done.
- A case-control interview study was carried out in Long Island 1996 to assess the relationship of breast cancer risk and residential proximity to industrial facilities including traffic. A number of post and pre-menopausal women in Nassau and Suffolk Counties in Long Island were observed. It was found that there was a significantly elevated risk of breast cancer among post-menopausal women who were subject to exposure from chemical facilities, however this elevated risk was not seen for premenopausal women. (Lewis-Michl et al. 1996)
- Furthermore, a study done in 2005 was similarly carried out to measure of the exposure to PAHs in early life, and if it were to lead to the occurrence of breast cancer. Total suspended solids were used as a measure of PAHs. As in the above study, it was found in post-menopausal women whom were exposed to high concentrations ( >140 microg/m(3) ) of TSPs had a higher risk (2.42 more likely) of developing breast cancer than compared to lower concentrations (<84 microg/m(3)). However results for premenopausal women were shown that actually exposure to PAHs at early age may cause a reduction in the risk of breast cancer. (Bonner et al. 2005)
- It has also been determined that many PAHs such as benzo[a]pyrene (BaP) are found within cigarette smokes. A case control study was done to assess smoking related DNA-adducts in breast tissue, and see if there is any correlation between their concentration and the incidence of breast cancer. To test this, benzo(a)pyrene diol-epoxide (BPDE)-induced mutagen sensitivity and polymorphisms of GSTM1 and GSTT1 were evaluated in a pilot case-control study of breast cancer. Blood samples were tested from 100 breast cancer patients and 100 controls. It was found that breast cancer patients had a higher frequency of chromatid breaks than controls, which was associated with a >3 times increase in the incidence of breast cancer. Therefore suggesting that sensitivity to BPDE-induced chromosomal aberrations may be a factor in developing breast cancer. (Silva et al. 2010) Furthermore there’s a sufficient amount of evidence that suggests there is a correlation between smoking and incidence of breast cancer. For example, smoking causes human bronchial cells to produce stress-induced unregulated cRNA transcripts. These transcripts can also be seen in breast cancer cell lines, therefore suggesting that smoking can up regulate ncRNAs in breast cancer. (Xiong et al. 2001)
Cohort Studies
A Cohort study is a study in which a group of samples/patients (cohorts) which share a common factor (age/race etc), are followed and evaluated with respect to an outcome/disease to discover what exposure characteristics to each cohort may be associated with the outcome/disease. (Himmelfard Health Science library, 2011)
- A cohort study was done with California teachers to measure the incidence of breast cancer in relation to hazardous air pollutants. This longitudinal study was carried out for 15 years, and from the initial 112,378 California Teachers Study participants, 5,676 women were diagnosed with invasive breast cancer. A modelled annual average ambient air concentration of MGCs from the US EPA were linked to cohorts addresses. And from this Cox proportional hazards model was used to find a 95% confidence interval of cohorts hazard ratio associated with their address. It was found that statistically significant associations between increased risk of breast cancer and residence in areas with high estimated ambient concentrations of notably propylene oxide, vinyl chloride along with PAHs. (Garcia et al. 2015)
- A cohort study was done to determine under identical conditions the relative carcinogenic potency in the mammary glands of female rats of benzo[a]pyrene amongst other carcinogens. Each 30 day old female rats were given BaP weekly for 8 weeks, other rats were given carcinogens such as 1-nitropyrene (1-NP) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). 41 weeks after the last carcinogen administration for the rats, they were killed and assessed for mammary gland tumours. Both malignant and cancerous mammary gland tumours were found for rats treated with BaP, the highest level found for all carcinogens tested. Within this test rats were directly given concentrations of BaP regularly over a number of weeks, this therefore would cause for immediate direct effects to be seen. Although this is not comparable for human subjects, as they wouldn’t be given such high dosages at regular intervals. However, this study does provide evidence that BaP can implicate breast cancer in humans and that further study should be done to assess this. (el-Bayoumy et al. 1995)
- Likely mechanisms in which PAHs exert their effect.
Polycyclic Aromatic Hydrocarbons as stated are Endocrine Disrupting Chemicals (EDCs). An EDC is defined by the US EPA as ‘‘an exogenous agent that interferes with synthesis, secretion, transport, metabolism, binding action, or elimination of natural blood-borne hormones that are present in the body and are responsible for homeostasis, reproduction, and developmental process’. (Diamanti-Kandarakis et al. 2009)
These ultimately can disrupt and alter endocrine processes and cause severe consequences by altering normal mammary tissue development. This has been shown in both rodent and human models. The mechanisms of EDCs such as PAHs are broader than anticipated and extend further than just exerting actions through nuclear hormone receptors. (el-Bayoumy et al. 1995, Diamanti-Kandarakis et al. 2009, Xiong et al. 2001)
Generally speaking, upon exploration of PAHs causing cancer in humans, it has been seen in many studies that tumors develop where PAHs have been administered to the test subject. i.e gastric tumours after oral administration, skin tumours after dermal application. However studies also show that PAHs, benzo(a)prune in particular when administered the oral route (mechanism of exposure being inhalation/ingestion), that tumours are seen in the lungs, bladder and mammary glands. This causes many different epigenetic effects.(Knower et al. 2014)
Epigenetic effects PAHs can have on breast tissue can include:
- Forming DNA adducts near methylation sites in breast epithelium and milk. Benzo[a]pyrene (BaP) metabolite BaP-diolepoxide (BPDE) has been found in breast milk. This therefore proves that these carcinogens penetrate ductal epithelial cells where most breast cancer is said to arise. (Knower et al. 2014, Gorlewska-Roberts et al. 2002)
- Altering DNA methylation and histone modification patterns. (Knower et al. 2014)
Upon the exploration of exact AOPs of PAH’s causing Breast Cancer, little information was found. However, what was found is that PAHs are xenobiotics. Xenobiotics metabolism occurs in the liver. Therefore, after ingestion/inhalation/adsorption of PAHs into the body, they bind to the lipid membranes and are transported by lipoproteins in the blood. Here, two main pathways can occur. (Ewa and Danuta 2017)
Phase I – three main pathways of activation can occur: (Ewa and Danuta 2017)
- formation of dihydrodiol epoxides catalysed by cytochrome P450 enzymes and epoxide hydrolase.
- formation of a PAH radical cation in a metabolic oxidation process by cytochrome P450 peroxidase activity.
- formation of ortho-quinones via oxidation of catechols by dihydrodiol dehydrogenase.
Phase II – following the introduction of a polar group, conjugating enzymes usually add endogenous substituents, such as sugars, sulphates, or amino acids. This therefore increases water solubility, which in turn makes them easy to excrete. Although this process is generally a detoxication sequence, reactive intermediates may sometimes prove to be much more toxic than the original compounds. (Ewa and Danuta 2017)
However the most common mechanism seen by the exposure of BaP, the most common PAH is as follows:
- In Phase I, oxidation reactions catalyzed by cytochrome P450 enzymes (CYPs) and hydroxylation occurs. The CYPs: CYP1A1 and CYP1B1 are highly inducible by the exposure to PAHs via the aryl hydrocarbon receptor (AhR). Once a complex is formed, AhR-PAH, a heat shock protein (Hsp90) is released. The complex is then translocated to the Hsp90’s nucleus. Here a heterodimer is formed with an AhR-Nuclear-Translocator (ARNT) which binds to DNA via the xenobiotic response element in the promoter region of the P450 genes. This therefore suggests a method by which PAHs assists in the role of tumorigenesis. (Ewa and Danuta 2017)
- How the PAHs effects are modified by interaction with other agents or interacts with genome to produce different problems for different genotypes.
PAHs carcinogenicity can be influenced by a variety of factors such as synergistic and antagonistic effects of other compounds which are emitted together with PAHs during incomplete combustion. (Barhoumi et al. 2014)
Most notably, a number of both in-vitro and in-vivo studies have shown that dietary supplementation with natural chemoprotective agents such as n-3 polyunsaturated fatty acids enhance anti-tumor action. Within an investigation in 2014, control fatty acids such as oleic acid (OA), linoleic acid (LA)) and docosahexaenoic acid (DHA) were used to test the effects on the uptake and metabolism of BaP in A549 cells. As stated above A549 cells activate BaP through the P450 system to form metabolites which can essentially prove to be carcinogenic. It was seen DHA treated cells showed significantly lower pyrene like metabolites, additionally DHA proved to have a lower abundance of carcinogen BaP 7,8-dihydrodiol and the 3-hydroxybenzo[a]pyrene metabolites compared to other treatments. Therefore proving that DHA supplements could potentially mediate BaP carcinogenic effects. (Barhoumi et al. 2014)
Conclusion
As stated various times within multiple reports and papers, the exact etiology of breast cancer in women is unknown.
However there is sufficient evidence to cause speculation as to if PAH implicates breast cancer in women. Various longitudinal studies have been carried out and there is evidence, as discussed above, to suggest that there’s a correlation between PAH concentration and the incidence of breast cancer. It has also been stated in various studies that the implication of PAHs causing breast cancer is higher in postmenopausal women than premenopausal. However so is the incidence of breast cancer.
There has been direct evidence to prove that PAH administration to rodents in studies can cause mammary gland tumours. However this does not directly relate to humans as the concentration of PAHs given to rodents would not directly correlate with what humans are exposed to.
In conclusion there is sufficient evidence to suggest PAHs cause breast cancer, and more direct studies need to be done.
References
Abdel-Shafy, H.I. and Mansour, M.S.M. 2016. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum, 25(1), pp.107–123.
Baird, W.M., Hooven, L.A. and Mahadevan, B. 2005. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environmental and Molecular Mutagenesis, 45(2–3), pp.106–114.
Barhoumi, R., Mouneimne, Y., Chapkin, R.S. and Burghardt, R.C. 2014. Effects of Fatty Acids on Benzo[a]pyrene Uptake and Metabolism in Human Lung Adenocarcinoma A549 Cells. PLoS ONE, 9(3).
el-Bayoumy, K., Chae, Y.H., Upadhyaya, P., Rivenson, A., Kurtzke, C., Reddy, B. and Hecht, S.S. 1995. Comparative tumorigenicity of benzo[a]pyrene, 1-nitropyrene and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine administered by gavage to female CD rats. Carcinogenesis, 16(2), pp.431–434.
Bonner, M.R., Han, D., Nie, J., Rogerson, P., Vena, J.E., Muti, P., Trevisan, M., Edge, S.B. and Freudenheim, J.L. 2005. Breast cancer risk and exposure in early life to polycyclic aromatic hydrocarbons using total suspended particulates as a proxy measure. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 14(1), pp.53–60.
Diamanti-Kandarakis, E., Bourguignon, J.-P., Giudice, L.C., Hauser, R., Prins, G.S., Soto, A.M., Zoeller, R.T. and Gore, A.C. 2009. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocrine Reviews, 30(4), pp.293–342.
Ewa, B. and Danuta, M.-Š. 2017. Polycyclic aromatic hydrocarbons and PAH-related DNA adducts. Journal of Applied Genetics, 58(3), pp.321–330.
Garcia, E., Hurley, S., Nelson, D.O., Hertz, A. and Reynolds, P. 2015. Hazardous air pollutants and breast cancer risk in California teachers: a cohort study. Environmental Health, 14.
Gorlewska-Roberts, K., Green, B., Fares, M., Ambrosone, C.B. and Kadlubar, F.F. 2002. Carcinogen-DNA adducts in human breast epithelial cells. Environmental and Molecular Mutagenesis, 39(2–3), pp.184–192.
Kim, K.-H., Jahan, S.A., Kabir, E. and Brown, R.J.C. 2013. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environment International, 60, pp.71–80.
Knower, K.C., To, S.Q., Leung, Y.-K., Ho, S.-M. and Clyne, C.D. 2014. Endocrine disruption of the epigenome: a breast cancer link. Endocrine-Related Cancer, 21(2), pp.T33–T55.
Korsh, J., Shen, A., Aliano, K. and Davenport, T. 2015. Polycyclic Aromatic Hydrocarbons and Breast Cancer: A Review of the Literature. Breast Care, 10(5), pp.316–318.
Lee, B.M. and Shim, G.A. 2007. Dietary Exposure Estimation of Benzo[a]pyrene and Cancer Risk Assessment. Journal of Toxicology and Environmental Health, Part A, 70(15–16), pp.1391–1394.
Lewis-Michl, E.L., Melius, J.M., Kallenbach, L.R., Ju, C.L., Talbot, T.O., Orr, M.F. and Lauridsen, P.E. 1996. Breast cancer risk and residence near industry or traffic in Nassau and Suffolk Counties, Long Island, New York. Archives of Environmental Health, 51(4), pp.255–265.
Morris, J.J. and Seifter, E. 1992. The role of aromatic hydrocarbons in the genesis of breast cancer. Medical Hypotheses, 38(3), pp.177–184.
Rundle, A., Tang, D., Hibshoosh, H., Estabrook, A., Schnabel, F., Cao, W., Grumet, S. and Perera, F.P. 2000. The relationship between genetic damage from polycyclic aromatic hydrocarbons in breast tissue and breast cancer. Carcinogenesis, 21(7), pp.1281–1289.
Silva, J.M., Perez, D.S., Pritchett, J.R., Halling, M.L., Tang, H. and Smith, D.I. 2010. Identification of long stress-induced non-coding transcripts that have altered expression in cancer. Genomics, 95(6), pp.355–362.
Study Design 101 – Case Control. Available from: https://himmelfarb.gwu.edu/tutorials/studydesign101/casecontrols.html [Accessed November 13, 2018].
Study Design 101 – Cohort Study. Available from: https://himmelfarb.gwu.edu/tutorials/studydesign101/cohorts.html [Accessed November 13, 2018].
Stults, W.P. and Wei, Y. 2018. Ambient air emissions of polycyclic aromatic hydrocarbons and female breast cancer incidence in US. Medical Oncology (Northwood, London, England), 35(6), p.88.
VanRooij, J.G., De Roos, J.H., Bodelier-Bade, M.M. and Jongeneelen, F.J. 1993. Absorption of polycyclic aromatic hydrocarbons through human skin: differences between anatomical sites and individuals. Journal of Toxicology and Environmental Health, 38(4), pp.355–368.
Xiong, P., Bondy, M.L., Li, D., Shen, H., Wang, L.E., Singletary, S.E., Spitz, M.R. and Wei, Q. 2001. Sensitivity to benzo(a)pyrene diol-epoxide associated with risk of breast cancer in young women and modulation by glutathione S-transferase polymorphisms: a case-control study. Cancer Research, 61(23), pp.8465–8469.
Cite This Work
To export a reference to this article please select a referencing style below:
Related Content
All TagsContent relating to: "cancer"
Cancer comprises several diseases that are characterized by abnormal cell growth. There are over one hundred varieties of cancer usually named for their cell or organ type. Cancer is the root cause of death across the world evidenced by WHO which established that around one in six deaths all over the world are due to cancer.
Related Articles


